Abstract
Statements About Intent to Share Individual Participant Data at ClinicalTrials.gov
Annice Bergeris,1 Tony Tse,1 Deborah A. Zarin1
Objective
Following recent calls for clinical trialists to declare their plans to share individual participant data (IPD) prior to study initiation, ClinicalTrials.gov added 2 optional data elements in December 2015: (1) Plan to Share IPD (submitted at study initiation) and (2) Available Study Data/Documents (submitted after study completion). We sought to characterize responses to ClinicalTrial.gov’s IPD sharing–related questions.
Design
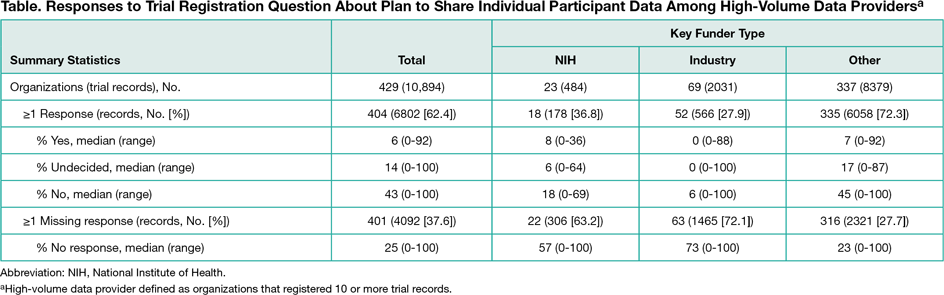
We summarized responses to IPD sharing–related questions for all interventional studies initially registered in 2016 and for the subset of trials registered by “high-volume data providers,” defined as organizations that registered 10 or more records. Organizations were categorized by key funder type, ie, were categorized as “NIH” if at least 1 National Institutes of Health institute was listed as a sponsor or collaborator, as “industry” if not classified as NIH and at least 1 company was listed as a sponsor or collaborator, and as “other” for all remaining records. Because of considerable heterogeneity in responses among the high-volume data provider subsample, we further characterized responses for trials registered by the top 10 high-volume data provider organizations within each key funder type.
Results
Of 21,310 trial records analyzed by May 10, 2017, 14,523 (68.2%) included a response to the question about plans to share IPD; 1930 records (13.3%) indicated yes, 3821 (26.3%) indicated undecided, and 8772 (60.4%) indicated no. Proportions within each key funder type varied among the 10,894 records from high-volume data providers (Table). Among the top 10 organizations within each key funder type, the percentage of records indicating that plans exist for sharing IPD ranged from 0% to 24% for NIH-funded studies, 0% to 63% for industry-funded studies, and 0% to 28% of studies with other funding. Among 131 records indicating that documents were available for sharing, 76 specified the study protocol would be shared, 52 specified other documents (eg, information leaflets), 32 specified informed consent forms, 16 specified the clinical study report, and 14 specified the individual participant data set. Five of 14 records (36%) specifying availability of IPD listed no plans for sharing IPD.
Conclusions
Sixty-eight percent of trial registrants responded to an optional question about plans to share IPD. Among those respondents, 13% said they would share data and another 26% were undecided. Of the 131 records indicating availability of documents for sharing, only 14 indicated that IPD were available. Considerable cultural and scientific changes will be necessary before the sharing of IPD and associated documents becomes part of routine practice by clinical researchers.
1ClinicalTrials.gov, National Library of Medicine, National Institutes of Health, Bethesda, MD, USA, dzarin@mail.nih.gov
Conflict of Interest Disclosures:
All authors work for ClinicalTrials.gov. Dr Zarin is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract.
Funding/Support:
Supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health.