Abstract
Frequency of Reporting on Patient Involvement in Research Studies Published in a General Medical Journal: A Descriptive Study
Amy Price,1,2 Sara Schroter,1 Rosamund Snow,1,3 Sophie Staniszewska,4,5 Sam Parker,1 Tessa Richards1
Objective
The requirements for planning of public involvement in research—ie, research “with” or “by” members of the public rather than “to,” “about,” or “for” them—within grant applications has increased. To date, there is not an agreed method of reporting public involvement in research, and this can make such involvement challenging to identify. To address this, The BMJ now asks submitting authors to include a dedicated section on how they involved patients in their research and, if they did not, to state there was no involvement. We explore the early influence on public involvement reporting, frequency, and practice following the introduction of a mandatory public involvement section.
Design
We report a before-and-after comparison of published research articles to assess whether the rate of reporting of public involvement in research increased with the introduction of a mandatory section for describing this involvement. Two researchers independently extracted data and reached consensus on incidences and types of public involvement in research across two 12-month samples. No study designs were excluded, because public involvement in research is possible with studies that have no direct contact with participants, eg, systematic reviews.
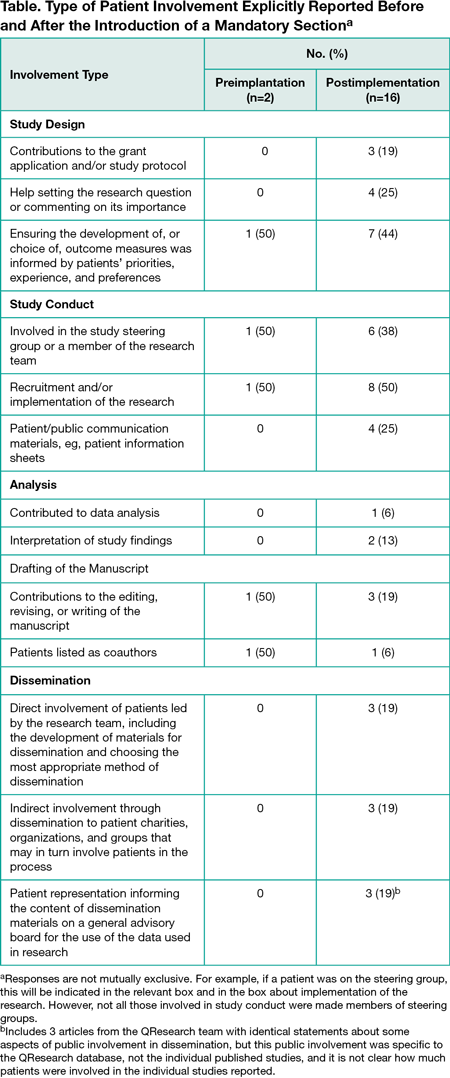
Results
Between June 1, 2013, and May 31, 2014, The BMJ published 189 research articles. Two (1.1%) reported public involvement activity. From June 1, 2015, to May 31, 2016, following the introduction of the public involvement section, The BMJ published 152 research articles, of which 16 (10.5%) reported public involvement. Patients were included in multiple aspects of research, from grant applications and study design to coauthorship and dissemination (Table). Of the 18 articles including some information on public involvement, 6 (33.3%) clearly acknowledged patients’ help or commented on the value of their contributions, and 2 (11.1%) included patient contributors as coauthors.
Conclusions
Public involvement in research is not commonplace, despite being encouraged by research funders. This is not solely a reporting issue, as the proportion of papers reporting public involvement was modest, even after introducing the mandatory public involvement declaration within the methods section of The BMJ articles. Some authors may have initiated their research prior to The BMJ mandatory public involvement reporting initiative, but some ethical review boards and funding agencies have been requesting this involvement for some years. Journals and funders should collaborate to improve guidance on how to involve and report patient involvement in research. Reporting innovative ways patients are involved in research processes may encourage practice in this important area.
1The BMJ, London, UK, dr.amyprice@gmail.com; 2Department of Continuing Education, University of Oxford, Oxford, UK; 3University of Oxford Medical School, Oxford, UK; 4Patient and Public Involvement and Experiences of Care Program, University of Warwick, Coventry, UK; 5Research Involvement and Engagement
Conflict of Interest Disclosures:
Sara Schroter, Rosamund Snow, Sam Parker, and Tessa Richards are employed by The BMJ, which has a patient partnership initiative. Amy Price, Rosamund Snow, and Tessa Richards are patients with long-term medical conditions and are committed to the involvement of patients in all stages of the research process. Amy Price is a research fellow at The BMJ. Sophie Staniszewska has no conflicts of interest.
Funding/Support:
We received no external funding for this study.
Additional Contributions:
Our powerful and inspiring coauthor, Rosamund Snow, passed away before this work could be published. We are thankful for her flexibility and steadfast leadership as we launched this initiative. We thank Sarah Foster (intern, The BMJ) for her help with the project.