Abstract
Age of Clinical Trial Data at the Time of Publication: A Systematic Review of Clinical Trials Published in 2015
John Welsh,1 Yuan Lu,1,2 Sanket S. Dhruva,3,4 Behnood Bikdeli,1,5 Nihar R. Desai,1,2 Liliya Benchetrit,2 Chloe O. Zimmerman,2 Lin Mu,2 Joseph S. Ross,1,3,6,7 Harlan M. Krumholz1,2,3,7
Objective
To determine the age of clinical trial data at the time of trial publication.
Design
Cross-sectional analysis of all clinical trials published in 2015 in Annals of Internal Medicine, BMJ, JAMA, JAMA Internal Medicine, Lancet, and New England Journal of Medicine. We determined the mid point of data collection to publication (age of data), in which the data collection period is defined as the start of enrollment to the end of follow-up. We also determined the days required for enrollment (enrollment time) and from the final study close-out visit to publication (dissemination time). We conducted multivariable linear regression models to identify factors associated with older data as well as longer enrollment and dissemination times.
Results
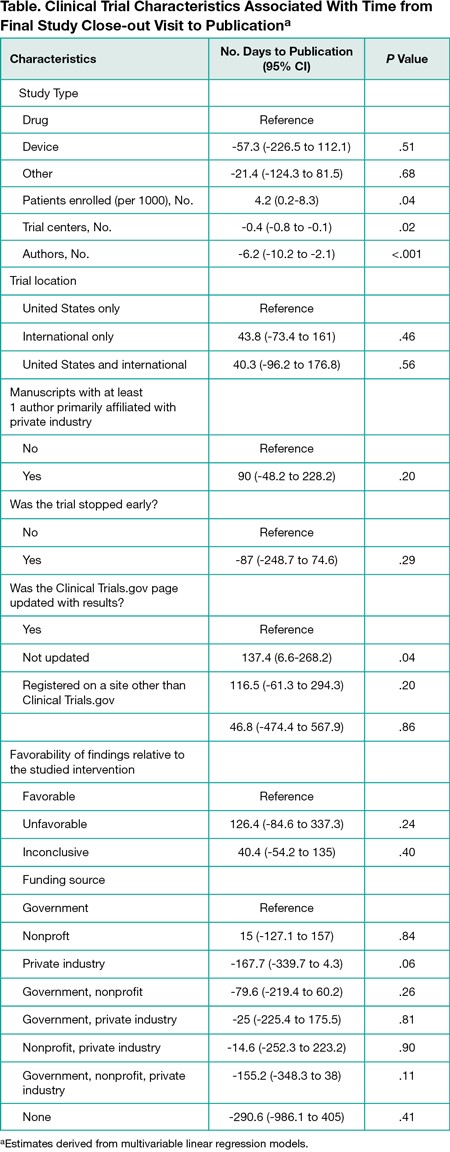
Among 341 clinical trials published in the 6 journals in 2015, 206 were drug trials (60.4%), 21 were device trials (6.2%), and 114 were trials of other interventions (33.4%). The median age of clinical trial data was 1032 days (interquartile range [IQR], 714.5-1408.5); 10% of trials represented practice from 5 years or more at the time of publication. Median enrollment duration was 798 days (IQR, 431.25-1285.5) or 1.37 days (IQR, 0.5-3.8) required per person enrolled. A median 451 days (IQR, 225-674) elapsed from final study close-out to publication with 60% of trials requiring more than 1 year to publish and 18.5% of trials requiring more than 2 years. In multivariable analyses, a larger number of patients, a smaller number of trial centers and authors, and results not updated on Clinical Trials.gov were statistically significantly associated with delays in publication because of the increased time required throughout 1 or more parts of a trial”s duration (Table).
Conclusions
By the time of publication, clinical trials in high-impact journals represent clinical practice with a median age of 1032 days, with 10% of trials representing practice from 5 or more years ago. There is substantial time required to publish a trial after the final study close-out.
1Center for Outcomes Research and Evaluation, Yale New Haven Health, New Haven, CT, john.welsh@yale.edu; 2Section of Cardiovascular Medicine, Department of Internal Medicine, Yale School of Medicine, New Haven, CT; 3Robert Wood Johnson Foundation Clinical Scholars Program, Department of Internal Medicine, Yale School of Medicine, New Haven, CT; 4Veterans Affairs Connecticut Healthcare System, West Haven, CT; 5Columbia University Medical Center/New York-Presbyterian Hospital, New York, NY; 6Section of General Medicine, Department of Internal Medicine, Yale School of Medicine, New Haven, CT; 7Department of Health Policy and Management, Yale School of Public Health, New Haven, CT, USA
Conflict of Interest Disclosures:
Drs Desai, Ross, and Krumholz are recipients of a research agreement from Johnson & Johnson through Yale University to develop methods of clinical trial data sharing. Drs Ross and Krumholz receive research support from Medtronic through Yale University to develop methods of clinical trial data sharing and through a grant from the US Food and Drug Administration and Medtronic to develop methods for postmarket surveillance of medical devices. Dr Ross receives research grant support from the Blue Cross Blue Shield Association. Dr Krumholz is the founder of Hugo, a personal health information platform, chairs a cardiac scientific advisory board for UnitedHealth, is a member of the advisory board for Element Science, and is a participant/participant representative of the IBM Watson Health Life Sciences advisory board.
Funding/Support:
This study received no external funding or support.