Abstract
The Prevalence of Conflict of Interest Disclosures in Biomedical Research
Quinn Grundy,1 Adam Dunn,2 Florence Bourgeois,3,4 Lisa A. Bero1
Objective
Conflict of interest disclosures are used to indicate a risk of bias in biomedical research but studies examining their prevalence are out of date or focused on narrow clinical topics. Our aim is to estimate the prevalence of conflict of interest disclosures in biomedical research across disciplines and determine article characteristics associated with higher rates of disclosure.
Design
We randomly sampled articles in Medline published from January 1, 2016, to December 31, 2016, in journals following the recommendations of the International Committee of Medical Journal Editors (ICMJE). There were no language restrictions. Non–peer-reviewed articles, including letters and news stories, were excluded. We developed a coding manual to classify the reported conflicts of interest and sources of study funding based on the National Academies of Medicine definition of conflict of interest and the ICMJE disclosure form. Independently, 2 researchers piloted the coding manual on a random sample, resolving discrepancies through verification and discussion.
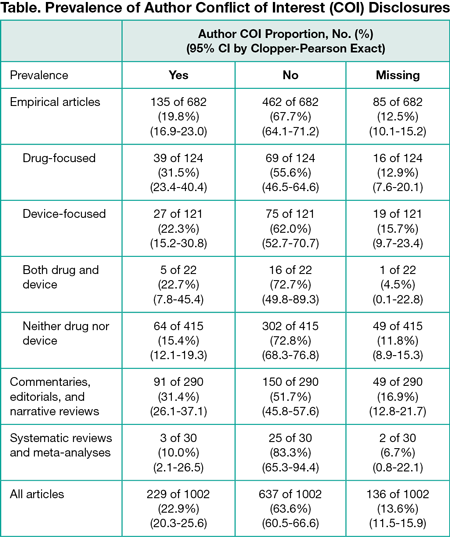
Results
After sampling 1650 articles, 1002 articles met our inclusion criteria. We found that 22.9% (95% CI, 20.3%-25.6%) disclosed a conflict of interest, 63.6% (95% CI, 60.5%-66.6%) disclosed no conflicts, and 13.6% (95% CI, 11.5%-15.7%) did not include a disclosure statement (Table). Articles focused on drugs, devices, or surgical procedures were significantly more likely to include authors with reported conflicts of interests (71 of 267 [26.6%]) than other empirical articles (64 of 415 [15.4%]) (difference, 11.2%; 95% CI, 3.3%-19.1%). Disclosure statements were inconsistent: we noted 130 different ways of stating there were no conflicts of interest, ranging from “None declared” to “Nothing to declare” to “No relevant conflicts” to statements 63 words long. Furthermore, 90 of 228 articles (39.4%) with statements contained extraneous biographical information not addressing conflicts of interest.
Conclusions
Many articles published in journals following the ICMJE recommendations fail to include disclosure statements. Just more than 1 in 5 biomedical articles report a relevant conflict of interest, which is generally consistent with a 2003 review that found that 23% to 28% of academic investigators receive funding from industry, suggesting this may be an underestimate. In current practice, conflict of interest statements are unstructured and inconsistently reported, precluding automatic extraction and analysis of conflict of interest statements.
1Charles Perkins Centre, Faculty of Pharmacy, The University of Sydney, Sydney, New South Wales, Australia; quinn.grundy@sydney.edu.au; 2Australian Institute of Health Innovation, Macquarie University, Sydney, New South Wales, Australia; 3Harvard Medical School, Harvard University, Cambridge, MA, USA; 4Division of Emergency Medicine, Boston Children’s Hospital, Boston, MA, USA
Conflict of Interest Disclosures:
Dr Bero is a Peer Review Congress Advisory Board Member but was not involved in the review or decision for this abstract.