Abstract
Augmenting Systematic Reviews With Information From ClinicalTrials.gov to Increase Transparency and Reduce Bias
Stacey Springs,1 Gaelen Adam,1 Thomas Trikalinos,1 John W. Williams Jr,2 Jennifer Eaton,2 Megan Von Isenburg,2 Jennifer M. Gierisch,2 Lisa M. Wilson,3 Ritu Sharma,3 Sydney M. Dy,3 Julie M. Waldfogel,3 Karen A. Robinson,3 Meera Viswanathan,4 Jennifer Cook Middleton,4 Valerie L. Forman-Hoffman,4 Elise Berliner,6 Robert M. Kaplan5,6
Objective
Prospective registration of clinical trials may improve transparency and reduce bias associated with selective reporting. Our objective was to evaluate the impact of access to and integration of information from ClinicalTrials.gov on the conclusions of systematic reviews in 5 clinical areas.
Design
Teams of systematic reviewers searched ClinicalTrials.gov for studies relevant to 5 different ongoing systematic reviews: Effectiveness of Treatment Options for the Prevention of Complications and Treatment of Symptoms of Diabetic Peripheral Neuropathy (DPN), Management of Infertility, Omega-3 Fatty Acids and Cardiovascular Disease, Strategies to Improve Mental Health Care for Children and Adolescents, and Tympanostomy Tubes in Children with Otitis Media. A semi-automated approach to matching studies using EndNote was not feasible owing to lack of standardization of format and location of the registry identification number in published reports. Teams compared trials, and information on trials, found from searches of other sources and determined whether information uniquely found in ClinicalTrials.gov changed confidence in evidence and review conclusion.
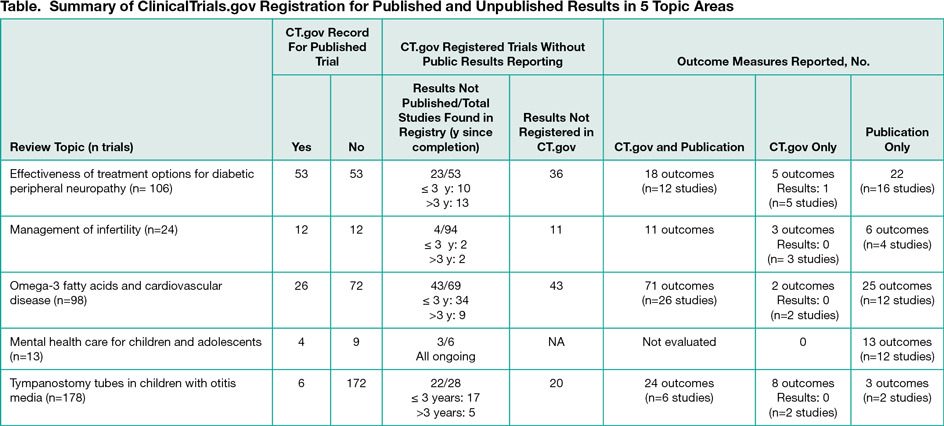
Results
Across all topics, 24% (101 of 419) of all included trials were registered in ClinicalTrials.gov; 38% (95 of 251 total registry records found) did not have results published in peer reviewed literature; and of trials with published and registry reported results, 63% (124 of 198) of outcomes matched in the publication and ClinicalTrials.gov records (Table) Despite the additional trials found in the searches of ClinicalTrials.gov, the strength of evidence and conclusions in each systematic review were unchanged, primarily owing to missing results of most of the additional trials found.
Conclusions
Across topic areas, only 24% (101 of 419) were registered in ClinicalTrials.gov and 38% (95 of 251) of studies did not have results published in peer-reviewed literature. The potential impact of this missing information on the conclusions of systematic reviews is unknown. When there were both ClinicalTrials.gov records and publications, 37% (74 of 198) of outcome measures did not match, raising a concern about bias owing to selective outcome reporting. It appears that prespecification of a primary outcome variable in ClinicalTrials.gov does not inhibit reporting other outcomes in publications. New rules requiring outcome measure specification and reporting should be considered. Journals and indexing tools could facilitate the inclusion of information from ClinicalTrials.gov into systematic review by adopting a more standardized format for listing the ClinicalTrials.gov identification number.
1Brown University Evidence Based Practice Center, Providence, RI, USA; 2Duke University Evidence Based Practice Center, Durham, NC, USA; 3Johns Hopkins University Evidence Based Practice Center, Baltimore, MD, USA; 4RTI/UNC Evidence Based Practice Center, Raleigh, NC, USA; 5Agency for Healthcare Research and Quality, Rockville, MD, USA, elise.berliner@ahrq.hhs.gov; 6Stanford University, Stanford, CA, USA
Conflict of Interest Disclosures:
None reported.
Funding/Support:
This project was funded by the Agency for Healthcare Research and Quality.
Role of the Funder/Sponsor:
Representatives from the Agency for Healthcare Research and Quality (AHRQ) served as a Contracting Officer’s Technical Representative and provided technical assistance during the conduct of the project and provided comments on draft versions of the project. AHRQ did not directly participate in the literature search, determination of study eligibility criteria, data analysis or interpretation.
Disclaimer:
The findings and conclusions in this abstract are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ and no statement in this abstract should be construed as an official position of AHRQ or the US Department of Health and Human Service.