Abstract
Types of Research Integrity Issues Encountered by a Specialist Research Integrity Group
Magdalena Morawska,1 Stephanie L. Boughton1
Objective
Data on the reasons why articles are retracted exist; however, the types and frequency of research integrity issues faced by editors day to day, particularly before publication, are unclear. Our objective was to categorize and determine the relative frequency of research integrity issues encountered by BioMed Central’s Research Integrity Group, which covers approximately 300 journals spanning biological and medical disciplines.
Design
We used a retrospective observational study design. We included all new inquiries regarding any aspect of research integrity sent to the Research Integrity Group between January 1, 2015, and December 31, 2016. The study period was chosen because it reflected a period when the structure and remit of the Group remained constant. The inquiries had been sent to the Research Integrity Group by editorial staff for advice and/or investigation of potential research and publication ethics issues following discussion with the journals’ editors in chief. They related to submitted manuscripts or published articles and may have been detected by the editors in chief, in-house staff, peer reviewers, or whistle-blowers. Editors in chief and editorial staff are not required to escalate all issues to the Group. We assigned each inquiry to 1 of 6 categories, adapted from the Committee on Publication Ethics (COPE) Case Taxonomy, covering different research integrity issues: authorship, competing interests, data issues, ethics/consent, peer-review process, and plagiarism/duplicate publication. Inquiries categorized as “ethics/consent” related to questions around ethics approval or consent for research involving human participants or consent for publication of potentially identifiable information (eg, case studies or images). We compared categories and their relative frequency for submitted manuscripts and published articles.
Results
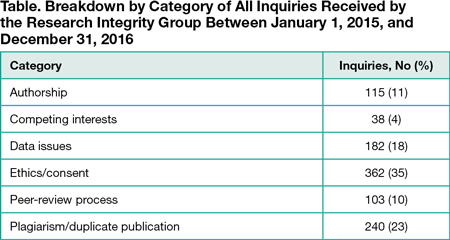
During the study period, the Research Integrity Group received 1040 inquiries: 690 (66%) related to submitted manuscripts and 350 (34%) to published articles. The Table shows the breakdown of inquiries by category. The largest category was ethics/consent (35%), and the second largest was plagiarism/duplicate publication (23%). For inquiries relating to submitted manuscripts only, almost half (49%) related to ethics/consent. The largest category for published articles was data issues (41%). These results have been used to inform training needs for both internal staff and external editors. Editorial policies and policy wording have also been revised in line with the results of this study.
Conclusions
Category frequency was different before and after publication. The high frequency of prepublication ethics/consent inquiries suggests that such issues can be detected at an early stage and that researchers need training to prevent such issues arising. Data issues were the most common for published articles, suggesting that problems with data may not always be detected by peer review and may only come to light after publication. Future studies could examine issues arising in nonbiomedical journals.
1Springer Nature, London, UK, stephanie.boughton@biomedcentral.com
Conflict of Interest Disclosures:
Both authors are employees of Springer Nature. At the time of data collection, Magdalena Morawska was an associate editor and Stephanie Boughton was a medical editor within BioMed Central’s Research Integrity Group (part of Springer Nature), the team dealing with the research and publication ethics inquires that were the subject of this study. Both are now part of Springer Nature’s Research Integrity Group.
Acknowledgments:
We thank the members of BioMed Central’s Research Integrity Group and Caroline Black for their helpful feedback on this abstract.