Abstract
Reporting of Sex and Gender in Clinical Trial Protocols and Published Results
Thiyagu Rajakannan,1 Kevin M. Fain,1 Rebecca Williams,1 Tony Tse,1 Deborah A. Zarin1
Objective
Biomedical research funders and journals have increasingly focused on the importance of assessing and reporting the effect of sex (biological factors) and gender (sociocultural factors) on health outcomes in clinical studies. Prior literature reviews have indicated that sex or gender are frequently not assessed or reported in published clinical studies. These studies did not assess research designs in protocols. The object of this study was to assess publicly available clinical study protocols and corresponding published studies to analyze how “sex” and “gender” information was incorporated in study design and reported.
Design
We identified a convenience sample of 80 articles from New England Journal of Medicine (NEJM) and JAMA published in 2014-2015 for which full protocols were available online. We then searched for and assessed the use of the terms “sex” and “gender” in the entire protocol and corresponding article.
Results
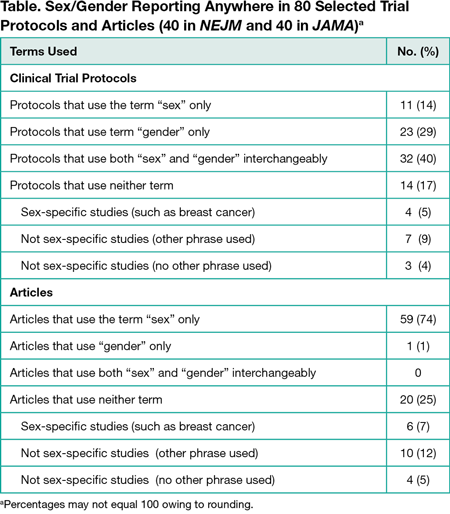
We found that, first, the terms “sex” and “gender” were not defined in any of the protocols or articles. Second, of the 80 clinical trials analyzed, 32 (40%) used both terms interchangeably in the protocol; 28 of these used “sex” only, and 4 used neither term in the corresponding article (Table). No article used the terms interchangeably. Finally, the term “gender” only was used in 23 (29%) protocols, but only 1 article used the term “gender.” Our data indicate imprecision in the use of the terms “sex” and “gender” in study protocols, suggesting a lack of appreciation among researchers of these distinct concepts. Articles generally used only “sex,” implying that journals enforce the use of specific and consistent terminology when reported. Of the 80 included studies, 14 (18%) articles did not use the terms “gender” or “sex” and were not sex-specific studies, of which 10 of these 14 (13% overall) used terms such as “men” or “women” but were unclear whether gender or sex was meant. We note the generalizability of these findings may be limited. These journals were chosen because they systematically included protocols for a large sample of clinical trials. The JAMA instructions for authors specifically addressed sex/gender reporting, although the NEJM author instructions did not explicitly address this reporting. Also, we did not assess how the constructs were used in the research.
Conclusions
Our study supports the need for continuing efforts to standardize the concepts of “sex” and “gender” and ensure their appropriate use in biomedical research.
1ClinicalTrials.gov, National Library of Medicine, National Institutes of Health, Bethesda, MD, USA, kevin.fain@nih.gov
Conflict of Interest Disclosures:
All authors report working on ClinicalTrials.gov. Dr Zarin is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision of this abstract.
Funding Support:
Supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health.
Role of the Funder:
All authors are full-time employees of the National Library of Medicine. The National Library of Medicine has approved this submission.