Registration of Observational Studies of Interventions: Prevalence, Characteristics, and Journal Policies
Abstract
Cecilie Jespersen,1,2 Zexing Song,3 An-Wen Chan,3,4 Asbjørn Hróbjartsson1,2
Objective
Observational studies of interventions use causal inference to assess the impact of interventions on health-related outcomes.1 Despite concerns about reporting bias, observational studies are not subject to the same registration requirements as clinical trials.2,3 We aimed to determine the prevalence of registration among published observational studies of interventions, assess the association between registration and study characteristics, analyze journal registration policies, and explore authors’ and editors’ attitudes about registration.
Design
We conducted a meta-epidemiologic cross-sectional study triangulating data from 4 sources. First, we searched PubMed for observational studies published in 2023. Eligible studies were cohort or case-control studies with a control group that assessed causal effects of health interventions. Corresponding registration information was collected. Second, authors of included studies were surveyed to explore reasons for and barriers to registration. Third, editorial policies were sampled from 40 journals: 20 sample-representative journals and the journals ranked in the top 20 in Journal Citation Reports by 2023 Journal Impact Factor across 8 specialty categories. Fourth, 1 editor per journal was invited to share their perspectives on registration. Primary outcomes were the prevalence of registered observational studies of interventions published in 2023 and the estimated association between registration and study characteristics, assessed by multivariable logistic regression. Sample size was estimated based on an expected 15% registration rate.
Results
Among 1100 screened studies, 200 were included: 69 and 128 cohort studies with prospective and retrospective data collection, respectively, and 3 case-control studies. In total, 28 (14%) were registered, and 17 of these (61%) were prospectively registered (<1 month of their start date) (Table 25-1069). Prospective design and protocol availability were positively associated with registration (retrospective vs prospective cohort: odds ratio [OR], 0.19 [95% CI, 0.07-0.54]; P = .002; no public protocol vs public protocol: OR, 0.04 [95% CI, 0.01-0.23]; P < .001). The survey response rate was 23% (46 responses); 60% of authors supported registration, although many only when registration was deemed relevant. Identified barriers included lack of journal requirements for registration (56%) and limited resources (62%). None of the journal policies explicitly required registration of observational studies of interventions, while 12 (30%) encouraged it. Journals that encouraged registration had a higher 2023 Journal Impact Factor and more frequently encouraged public protocols. Editors had divergent opinions on registration. While some considered it to be worthwhile, just as many questioned the added value.
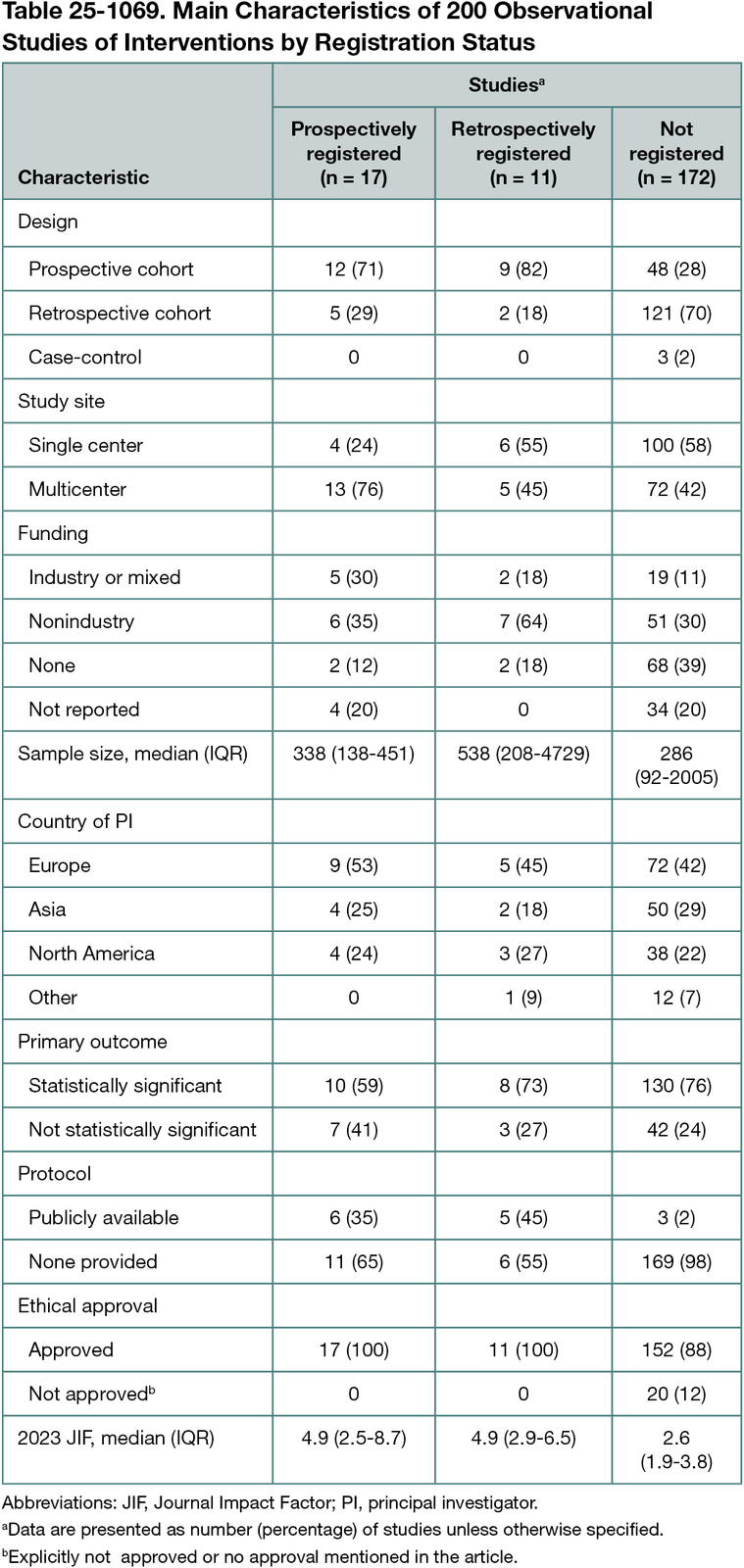
Conclusions
Only 1 in 7 contemporary observational studies of interventions were registered, although more often in cohort studies with prospective data collection and studies with a publicly available protocol. Authors identified the lack of journal requirements to registration as a key registration barrier, and only one-third of journals had supportive policies. Clearer guidance and journal policies on registration relevance (discriminating hypothesis-testing and hypothesis-generating studies) may reduce the risk of reporting biases in observational studies of interventions.
References
1. Hernán MA, Wang W, Leaf DE. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328(24):2446-2447. doi:10.1001/jama.2022.21383
2. Williams RJ, Tse T, Harlan WR, Zarin DA. Registration of observational studies: is it time? CMAJ. 2010;182(15):1638-1642. doi:10.1503/cmaj.09225
3. Leducq S, Zaki F, Hollestein LM, et al. The majority of observational studies in leading peer-reviewed medicine journals are not registered and do not have a publicly accessible protocol: a scoping review. J Clin Epidemiol. 2024;170:111341. doi:10.1016/j.jclinepi.2024.111341
1Cochrane Denmark & Centre for Evidence-Based Medicine Odense (CEBMO), University of Southern Denmark, Odense, Denmark, ceciliejespersen@health.sdu.dk; 2Open Patient data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark; 3Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; 4Women’s College Research Institute, Dept. of Medicine, University of Toronto, Toronto, Ontario, Canada.
Conflict of Interest Disclosures
An-Wen Chan is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract. No other disclosures were reported.
Acknowledgments
We thank all researchers who participated in the author survey for their valuable contribution to the findings of this study.
