Public Availability of Randomized Clinical Trial Protocols: A Repeated Cross-Sectional Study
Abstract
Christof Manuel Schönenberger,1 Malena Chiaborelli,1 Ala Taji Heravi,1 Lukas Kübler,2 Pooja Gandhi,3 Zsuzsanna Kontar,4 Julia Hüllstrung,1 Mona Elalfy,1 Jan Glasstetter,1 Dmitry Gryaznov,5 Belinda von Niederhäusern,6 Anette Blümle,7 Jason W. Busse,8 Szimonetta Lohner,4 Sally Hopewell,9 Alexandra Griessbach,1 Matthias Briel,1,10 Benjamin Speich1
Objective
Making protocols of randomized clinical trials (RCTs) publicly available is important for the trustworthiness and quality of medical research.1 In a previous study, only 36% of RCTs that received ethical approval in 2012 had a publicly available protocol.2 Repeating this study with RCTs approved in 2016, we aimed to generate current evidence on the availability of RCT protocols, sources where they are shared, and changes over time.
Design
Using a sample of RCTs receiving ethical approval in 2016 in Switzerland, Canada, Germany, or the UK,3 we investigated the number of available protocols. For all RCTs, we checked if a result publication was available by searching PubMed, Google Scholar, trial registries, and Google. We extracted baseline characteristics for all RCTs. Up to June 2024, we systematically searched for (1) protocols available as peer-reviewed publications, (2) protocols attached to trial registries, and (3) protocols shared with result publications of RCTs. Multiple sources per protocol were possible. All searches and data extraction were performed in duplicate. We used multivariable logistic regression to examine the association of protocol availability with trial characteristics such as sample size, drug vs nondrug interventions, multicenter vs single-center status, and RCT approval in 2016 vs 2012.
Results
Of 347 included RCTs, 228 (65.7%) had an available protocol (Table 25-0957); 150 protocols (43.2%) were available as files on trial registries, 91 (26.2%) as supplementary material to a result publication, and 81 (23.3%) as peer-reviewed publications. Protocol availability improved over time (65.7% in 2016 vs 36.2% in 2012), particularly in industry trials (83.4% in 2016 vs 34.6% in 2012), but improved little in nonindustry trials (46.4% 2016 vs 38.1% 2012). In a regression model with pooled data from trials approved in 2012 and approved in 2016, trials with a medium sample size (100-500 participants) (odds ratio [OR], 2.12; 95% CI, 1.32-3.43; P = .001), trials with a large sample size (>500 participants) (OR, 6.12; 95% CI, 3.45-11.07; P < .001), and multicenter trials (vs single-center trials) (OR, 2.66; 95% CI, 1.59-4.52; P < .001) showed significantly higher odds of having an available protocol. A significant interaction term indicated dependence of sponsorship and year (OR, 0.13; 95% CI 0.06-0.27; P < .001).
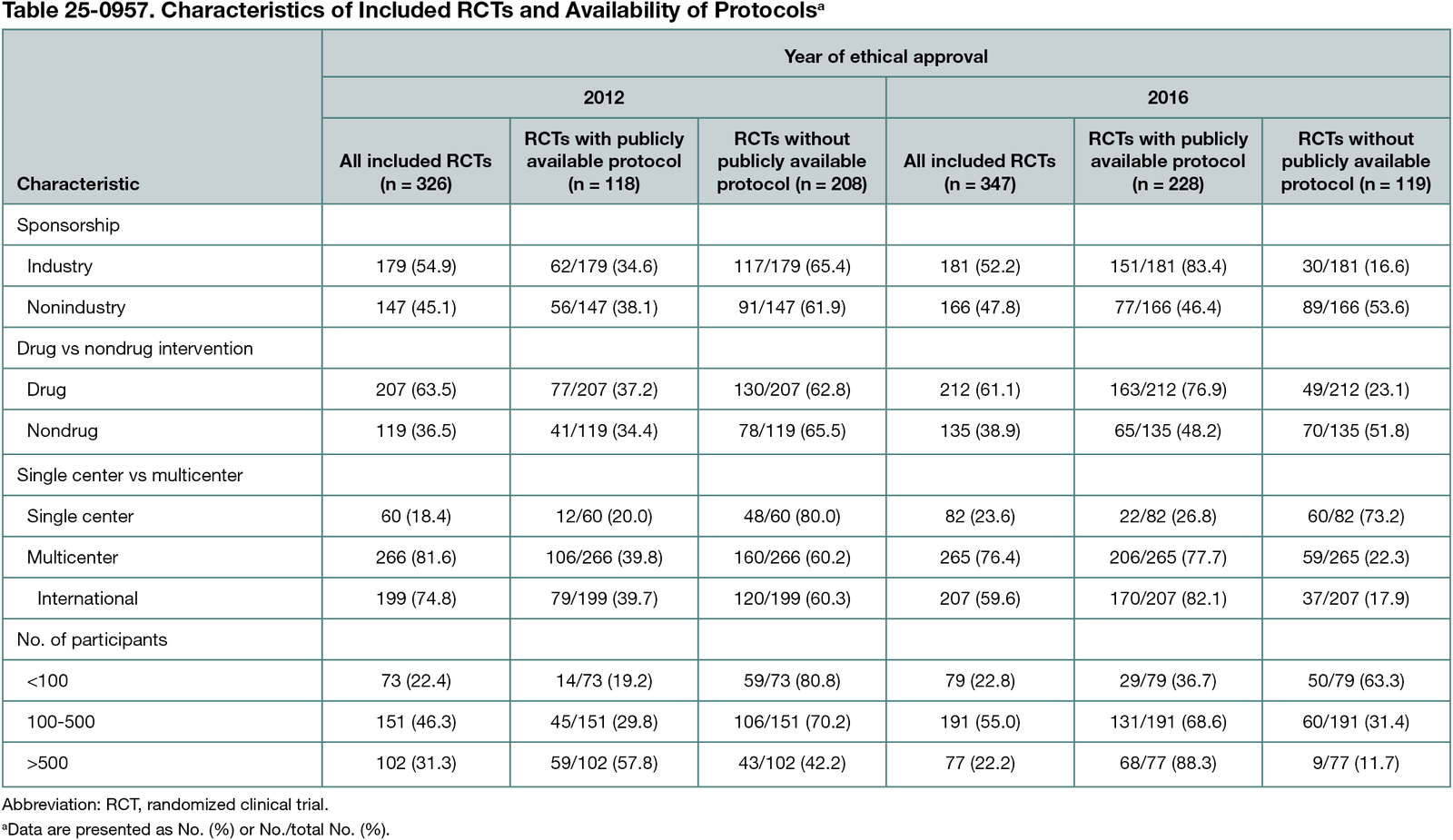
Conclusions
The availability of protocols increased in RCTs approved in 2016 compared with RCTs approved in 2012. This was mainly driven by industry-sponsored trials and may be explained by updated US regulations. Since 2017, all trials investigating drugs or devices that are or are intended to be approved, licensed, or cleared by the US Food and Drug Administration must have a publicly available protocol. Efforts to further improve protocol availability should be continued, especially in nonindustry-sponsored RCTs.
References
1. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257-266. doi:10.1016/S0140-6736(13)62296-5
2. Schönenberger CM, Griessbach A, Taji Heravi A, et al. A meta-research study of randomized controlled trials found infrequent and delayed availability of protocols. J Clin Epidemiol. 2022;149:45-52. doi:10.1016/j.jclinepi.2022.05.014
3. Gryaznov D, Odutayo A, von Niederhäusern B, et al. Rationale and design of repeated cross-sectional studies to evaluate the reporting quality of trial protocols: the Adherence to Spirit Recommendations (ASPIRE) study and associated projects. Trials. 2020;21(1):896. doi:10.1186/s13063-020-04808-y
1Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland, christofmanuel.schoenenberger@usb.ch; 2Department of Biomedicine, University Hospital Basel, University of Basel, Basel, Switzerland; 3University Health Network, Toronto, Ontario, Canada; 4MTA-PTE Lendület “Momentum” Evidence in Medicine Research Group, Department of Public Health Medicine, Medical School, University of Pécs, Pécs, Hungary; 5Viatris Innovation GMBH, Allschwil, Switzerland; 6Roche Pharma AG, Grenzach-Wyhlen, Germany; 7Clinical Trials Unit, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany; 8Department of Anesthesia, McMaster University, Hamilton, Ontario, Canada; 9Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK; 10Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Conflict of Interest Disclosures
None reported.
Funding/Support
This project was supported by the Swiss Federal Office of Public Health (to Matthias Briel). Christof Manuel Schönenberger was funded by the Janggen Pöhn Foundation and the Swiss National Science Foundation (MD-PhD grant number 323530_221860).
Role of the Funder/Sponsor
The funders had no role in the conduct of the study, the analysis of the data, or the writing of the abstract.
Acknowledgment
We thank all participating research ethics committees from Germany (Freiburg), Switzerland (Basel, Bellinzona, Bern, Geneva, Lausanne, St Gallen, Frauenfeld, and Zurich), Canada (Hamilton), and the UK (National Health Service Health Research Authority) for their support and cooperation.
