Metrics of Primary and Secondary Publications of Clinical Trials With Data Shared on the YODA Project Platform
Abstract
Erfan Taherifard,1 Hollin R. Hakimian,1 Maryam Mooghali,1 Sahil R. Mane,1 Mengyuan Fu,1 Stephen Bamford,2 Karla Childers,3 Nihar R. Desai,4 Cary P. Gross,1,5,6 Debbie Hewens,2 Harlan M. Krumholz,4,7,8 Richard Lehman,9 Jessica D. Ritchie,7 Tamsin Sargood,2 Joshua D. Wallach,10 Molly K. Willeford,7 Joseph S. Ross1,7,8
Objective
Sharing clinical trial data can advance scientific knowledge, maximize research impact, and reduce duplication, costs, and risks.1 However, the impact of such initiatives has not been systematically evaluated. The Yale Open Data Access (YODA) Project is an academic-based data-sharing initiative housed at Yale University.2 This study evaluated the impact of studies published using data from clinical trials sponsored by Johnson & Johnson that are shared on the YODA Project platform, distinguishing between studies led by the primary study investigators and external investigators.
Design
We conducted a cross-sectional study of clinical trials sponsored by Johnson & Johnson that were shared on the YODA Project platform as of December 31, 2021; trials without a primary publication were excluded. Primary publications—defined as the first full-length peer-reviewed published article reporting the trial’s primary end point—were identified. Secondary publications—defined as peer-reviewed published studies using individual-level participant data—were retrieved by screening citations to each trial’s primary publication in the Web of Science. The primary searches in the Web of Science were conducted from September 15 to December 15, 2024, with all searches updated as of December 15, 2024. We collected metrics for primary and secondary publications, including Impact Factors of publishing journals and annual citation counts, Altmetric scores, and Mendeley reader counts. Annual metrics were calculated by dividing each metric by the number of days since publication, then multiplying by 365. Secondary publications were categorized as internal (authored by primary study investigators) or external. Comparisons were performed using Mann-Whitney U tests.
Results
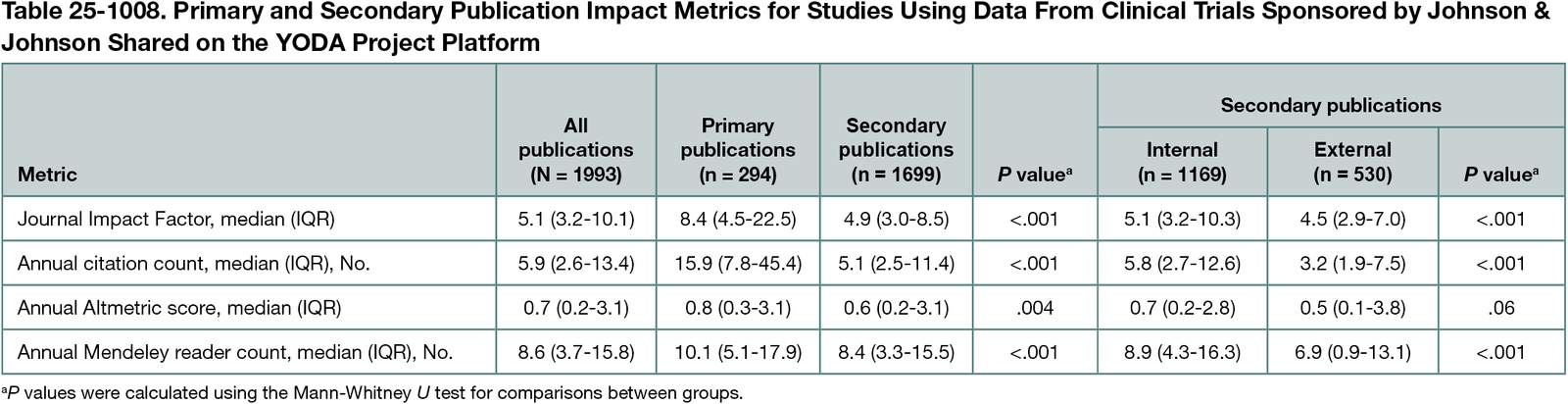
There were 294 trials with a primary publication sharing data on the YODA Project platform, 218 (74.1%) of which had at least 1 secondary publication, totaling 1699 secondary publications. Most were pooled analyses (1302 [76.6%]), as opposed to secondary analyses (397 [22.4%]). Trials of heart and blood disease interventions had the highest number of secondary publications per trial (median [IQR], 10.5 [2-19] publications). Compared with primary publications, secondary publications were published in journals with lower median (IQR) journal Impact Factors (8.4 [4.5-22.5] vs 4.9 [3.0-8.5]) and had lower annual citation counts (15.9 [7.8-45.4] vs 5.1 [2.5-11.4]), Altmetric scores (0.8 [0.3-3.1] vs 0.6 [0.2-3.1]), and Mendeley reader counts (10.1 [5.1-17.9] vs 8.4 [3.3-15.5]) (Table 25-1008). Secondary publications were predominantly internal (1169 [68.8%]), which, when compared with external secondary publications, were published in journals with higher median (IQR) Impact Factors (5.1 [3.2-10.3] vs 4.5 [2.9-7.0]) and had higher annual citation counts (5.8 [2.7-12.6] vs 3.2 [1.9-7.5]), Altmetric scores (0.7 [0.2-2.8] vs 0.5 [0.1-3.8]), and Mendeley reader counts (8.9 [4.3-16.3] vs 6.9 [0.9-13.1]).
Conclusions
Clinical trial data sharing through the YODA Project has fostered substantial scholarship, with nearly one-third of secondary studies published using these trials’ data by external investigators not affiliated with the primary study team. While secondary publications generally had lower impact metrics than primary publications, they still demonstrated meaningful scientific dissemination and usage, underscoring their contributions to the broader medical research enterprise.
References
1. Angraal S, Ross JS, Dhruva SS, Desai NR, Welsh JW, Krumholz HM. Merits of data sharing: the Digitalis Investigation Group trial. J Am Coll Cardiol. 2017;70(14):1825-1827. doi:10.1016/j.jacc.2017.07.786
2. Ross JS, Waldstreicher J, Bamford S, et al. Overview and experience of the YODA Project with clinical trial data sharing after 5 years. Sci Data. 2018;5:180268. doi:10.1038/sdata.2018.268
1Section of General Internal Medicine, Yale School of Medicine, New Haven, CT, US, erfan.taherifard@yale.edu; 2Johnson & Johnson, London, England, UK; 3Johnson & Johnson, New Brunswick, NJ, US; 4Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT, US; 5Cancer Outcomes, Public Policy, and Effectiveness Research Center, Yale School of Medicine, New Haven, CT, US; 6Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, CT, US; 7Yale–New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, CT, US; 8Department of Health Policy and Management, Yale School of Public Health, New Haven, CT, US; 9Institute of Applied Health Research, University of Birmingham, Birmingham, England, UK; 10Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, US.
Conflict of Interest Disclosures
Erfan Taherifard is an academic editor of PLOS One. Stephen Bamford, Karla Childers, Debbie Hewens, and Tamsin Sargood are current employees and shareholders of Johnson & Johnson. Nihar R. Desai, Cary P. Gross, Harlan M. Krumholz, Jessica D. Ritchie, Joshua D. Wallach, Molly K. Willeford, and Joseph S. Ross are members of the YODA Project leadership team and receive support from Johnson & Johnson to support data sharing through the YODA Project. Nihar R. Desai reported working under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay-for-performance programs and receiving research grants from and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Cytokinetics, Merck, Novartis, scPharmaceuticals, and Vifor. Cary P. Gross has received research funding from the NCCN Foundation (AstraZeneca) and Genentech and is an associate editor of JAMA Internal Medicine. Harlan M. Krumholz reported receiving options for Element Science and Identifeye and payments from F-Prime for advisory roles, being a cofounder of and holding equity in Hugo Health, Refactor Health, and ENSIGHT-AI, being associated with research contracts through Yale University from Janssen, Kenvue, and Pfizer, and being editor in chief of JACC. Jessica D. Ritchie reported receiving support from the US Food and Drug Administration (FDA) and Arnold Ventures. Joshua D. Wallach reported receiving support from the FDA and Arnold Ventures, having previously served as a consultant to Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC, and being an associate editor of JACC. Joseph S. Ross reported receiving support from the FDA, Arnold Ventures, Agency for Healthcare Research and Quality, and National Heart, Lung, and Blood Institute and being an expert witness at the request of the relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc that was settled in September 2022, and being a deputy editor of JAMA.
Funding/Support
This work was supported in part by a research grant from Johnson & Johnson through Yale University to establish a platform for clinical trial data sharing.
Role of the Funder/Sponsor
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the abstract; or decision to submit the abstract for presentation.
