Medical Journal Policies on Requirements for Clinical Trial Registration, Reporting Guidelines, and Data Sharing: A Systematic Review
Abstract
Kyobin Hwang,1 Zexing Song,2,3 Marsida Stafa,3 Jodie Chiu,4 An-Wen Chan1,3,5
Objective
We aimed to determine how often medical journals have policies requiring clinical trial registration, adherence to reporting guidelines, and participant-level data sharing. We also evaluated associations between journal characteristics and the existence of such policies for clinical trial manuscripts.
Design
A PubMed search using the clinical trial filter was conducted to identify journals that published at least 20 trials in 2023. We extracted publicly available data from journal websites, including policies on trial registration, adherence to reporting guidelines, trial protocol submission requirements, and availability of participant-level data. A practice was classified as required if the policy used words such as must, need, or should. Policies using language such as encouraged and preferred were classified as recommended practices. For each journal, we recorded the 2023 Clarivate impact factor, journal scope (general vs specialty), and publishing model (purely open access vs other). We calculated descriptive statistics to summarize the prevalence of transparency policies and used multivariable logistic regression to assess the association between journal characteristics and policy requirements.
Results
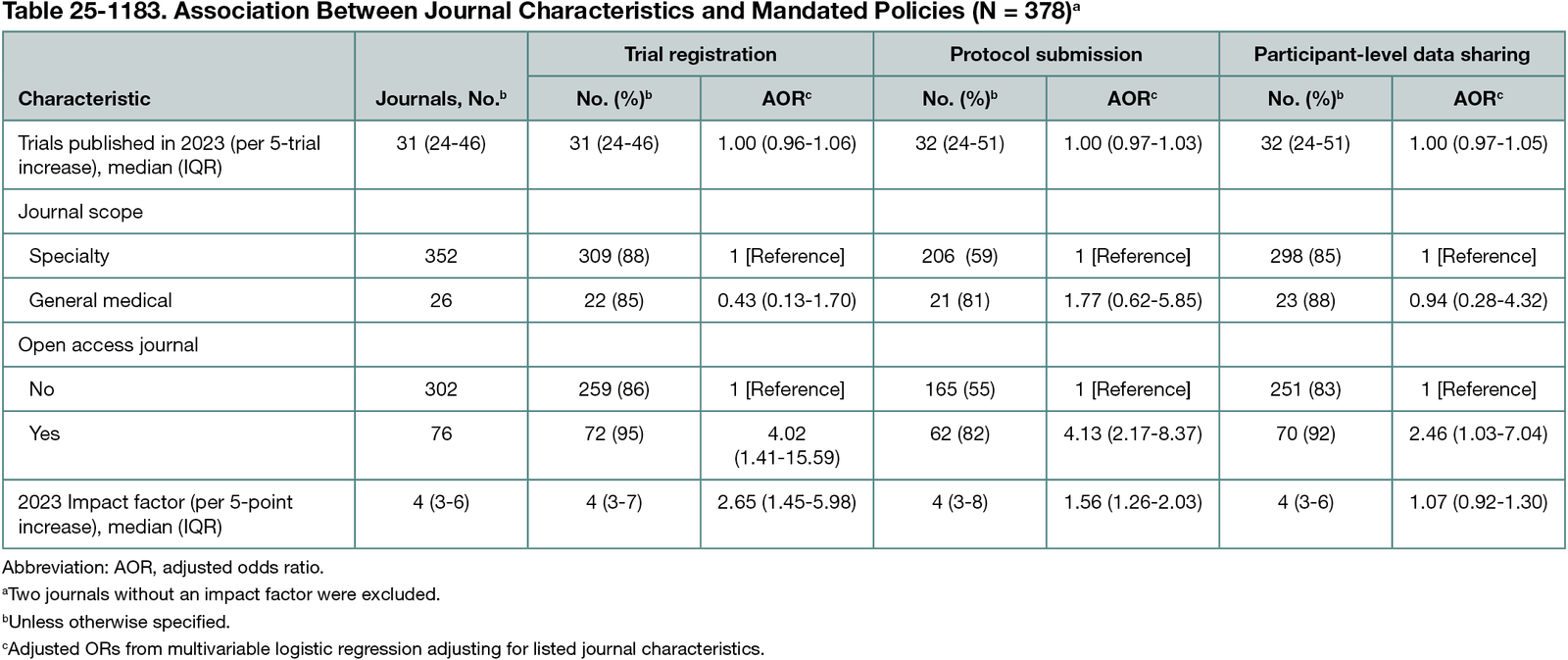
Among 380 included journals, 320 (84%) required trial registration, 11 (3%) recommended it, and 49 (13%) did not mention it. Adherence to a reporting guideline for clinical trials was required by 251 journals (66%), recommended by 74 (19%), and not mentioned by 55 (14%). Trial protocol submission was required by 118 (31%), recommended by 110 (29%), and not mentioned in 152 (40%). Public availability of participant-level datasets was required by 104 (27%), recommended by 218 (57%), and not mentioned by 58 (15%). A description of the data sharing plan was required by 212 journals (55.8%), recommended by 110 (28.9%), and not mentioned by 58 (15.3%). Purely open access journals had a 4-fold higher odds of requiring trial registration (adjusted odds ratio [AOR], 4.02; 95% CI, 1.41-15.59) and protocol submission (AOR, 4.13; 95% CI, 2.17-8.37) and 2.5 times higher odds of requiring public sharing of participant-level data (AOR, 2.46; 95% CI, 1.03-7.04) compared with other journals (Table 25-1183). Each 5-point increase in journal impact factor was associated with a more than 2.5-fold increase in the odds of requiring trial registration (AOR, 2.65; 95% CI, 1.45-5.98) and over 50% increase in the odds of requiring protocol submission (AOR, 1.56; 95% CI, 1.26-2.03). Impact factor was not significantly associated with requiring data sharing. No significant associations were found for journal scope or volume of trials published.
Conclusions
Journal policy requirements vary substantially in supporting best practices for clinical trial transparency. Journals with a purely open access publishing model and higher impact factor were more likely to adopt transparency policies. These findings highlight the need for improved editorial standards across the publishing landscape to promote transparency and reduce research waste.
1Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada, anwen.chan@utoronto.ca; 2Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; 3Women’s College Research Institute, Toronto, Ontario, Canada; 4Faculty of Health Science, University of Western Ontario, London, Ontario, Canada; 5Division of Dermatology, Department of Medicine, University of Toronto, Toronto, Ontario, Canada.
Conflict of Interest Disclosures
An-Wen Chan is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract.
