Abstract
Frequency and Format of Clinical Trial Results Disseminated to Participants: A Survey of Trialists
Sara Schroter,1 Amy Price,1,2 Mario Malički,3 Rosamund Snow,1,4 Tessa Richards,1 Mike Clarke5,6,7
Objective
Dissemination of research findings is central to research integrity and supports the translation of clinical knowledge into practice. This survey investigates the frequency and format of research dissemination to trial participants and patient groups and explores how patients are involved in determining the content and method of dissemination.
Design
First authors of clinical trials indexed in PubMed and published in English in 2014-2015 were emailed and invited to complete a SurveyMonkey questionnaire.
Results
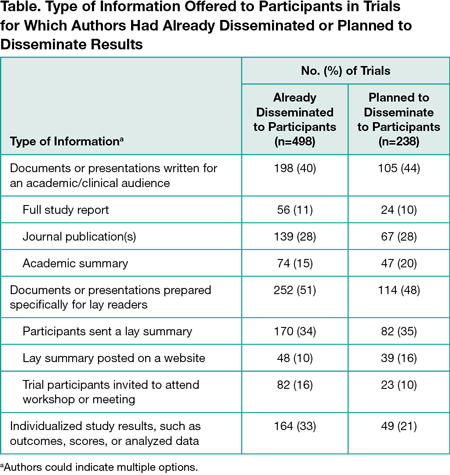
Surveys were sent to authors of 19,824 trials; 3227 responses were received (16%). Of the 3227 trials, 2690 had human participants and 1818 enrolled individual patients. Among the 1818, 906 authors (50%) had asked patients if they wanted to receive results, 305 (17%) had involved or planned to involve patients in developing dissemination materials, and 295 (16%) had involved or planned to involve patients in identifying appropriate dissemination methods. Four hundred ninety-eight (27%) reported that they had already disseminated results to participants and another 238 (13%) planned to do so, 600 (33%) did not plan to, 176 (10%) were unsure, and 256 (14%) responded with “other” or did not answer. Of the 498 authors who disseminated results to participants, 198 (40%) shared academic reports, 252 (51%) shared lay reports, and 164 (33%) provided individualized study results (Table). Among the 1818 trials, 577 authors (32%) shared or planned to share results with patients outside their trial by direct contact with charities/patient groups, 401 (22%) via informal patient communities, 845 (46%) via presentations at conferences with patient representation, 494 (27%) via mainstream media, and 708 (39%) by publishing lay summaries online. Relatively few authors of the 1818 trials reported that dissemination to participants and other patient groups was suggested to them by institutional bodies: by research funders (314 [17%] suggested dissemination to trial participants and 252 [14%] suggested dissemination to other patient groups), and by ethical review boards (333 [18%] suggested dissemination to trial participants and 148 [8%] suggested dissemination to other patient groups). Author-reported barriers to dissemination included not having access to patient contact information, insufficient funding and support, inaccessible patient groups (eg, deceased, vulnerable, or mobile), time interval between the study and publication, and lack of patient-centered dissemination training.
Conclusion
Fewer than half of respondents had disseminated or planned to disseminate their results to patients and only half of those in language tailored to patients. Motivation to disseminate appears to arise within research teams rather than from institutional bodies. Multiple factors need to be understood and overcome to facilitate wider, more effective dissemination of research to patients.
1The BMJ, London, UK, sschroter@bmj.com; 2Department of Continuing Education, University of Oxford, Oxford, UK; 3School of Medicine, University of Split, Split, Croatia; 4University of Oxford Medical School, Oxford, UK; 5Northern Ireland Methodology Hub, Belfast, UK; 6Northern Ireland Clinical Trials Unit, Belfast, UK; 7Centre for Public Health, Queen’s University Belfast, Belfast, UK
Conflict of Interest Disclosures:
Sara Schroter, Rosamund Snow, and Tessa Richards are or were employed by The BMJ, which has a patient partnership initiative. Amy Price, Rosamund Snow, and Tessa Richards are or were patients with long-term medical conditions and committed to increasing the dissemination of results to patients. Amy Price is a Research Fellow at The BMJ. Mario Malički has no conflicts of interest. Mike Clarke has been involved in many clinical trials and systematic reviews both as an organizer of the dissemination of his own research and using material disseminated by others. He seeks funding for these trials and reviews as well as for research into methodology, including dissemination.
Additional Information:
Rosamund Snow is deceased.
Funding/Support:
No external funding was received for this study. Mario Malički is partially funded by Croatian Science Foundation grant IP-2014-09-7672 Professionalism in Health Care.
Acknowledgments:
We honor Rosamund Snow, BMJ Patient Editor, who passed away before this work could be published. We applaud her insightful perspective, faithful diligence, and humor as we worked together on this research. We thank the 3 members of the BMJ Patient Panel for their comments on the survey content and the volunteers who pilot tested the survey, helped to improve the survey questions, and commented on the survey design.