Abstract
Factors Affecting Publication of Pediatric Intervention Trials
Sumaira Khalil,1 Devendra Mishra,2 Dheeraj Shah1
Objective
To assess the publication status and factors associated with subsequent publication of all pediatric intervention trials registered in the Clinical Trial Registry of India (CTRI) over a period of 5 years.
Design
This cross-sectional study was conducted from December 2021 to February 2022 and included the first 100 pediatric intervention trials registered in the CTRI from 2008 to 2012. Registry records were identified from the CTRI website using the keywords pediatric, paediatric, children, adolescent, infant, newborn, neonate, kids, and school. Trial characteristics (eg, blinding, type of intervention and comparator, setting, funding, source of funding, single or multicentric, and postgraduate thesis) were abstracted from each registered trial. A list of all the randomized clinical trials registered on the website was made and their subsequent publication was systematically searched on PubMed and Google Scholar using their registered CTRI number up to December 2021. For trials that were not found, repeat searches were performed, searching by the first author’s name, second author’s name, and title of the registered trial. The proportion of trials subsequently published and the time to publication from the date of registration were analyzed. Factors associated with publication were compared between trials that were published and those not published using χ² test and univariate analysis by calculating the odds ratio and 95% CI. Multivariable logistic regression was conducted for factors with P < .50.
Results
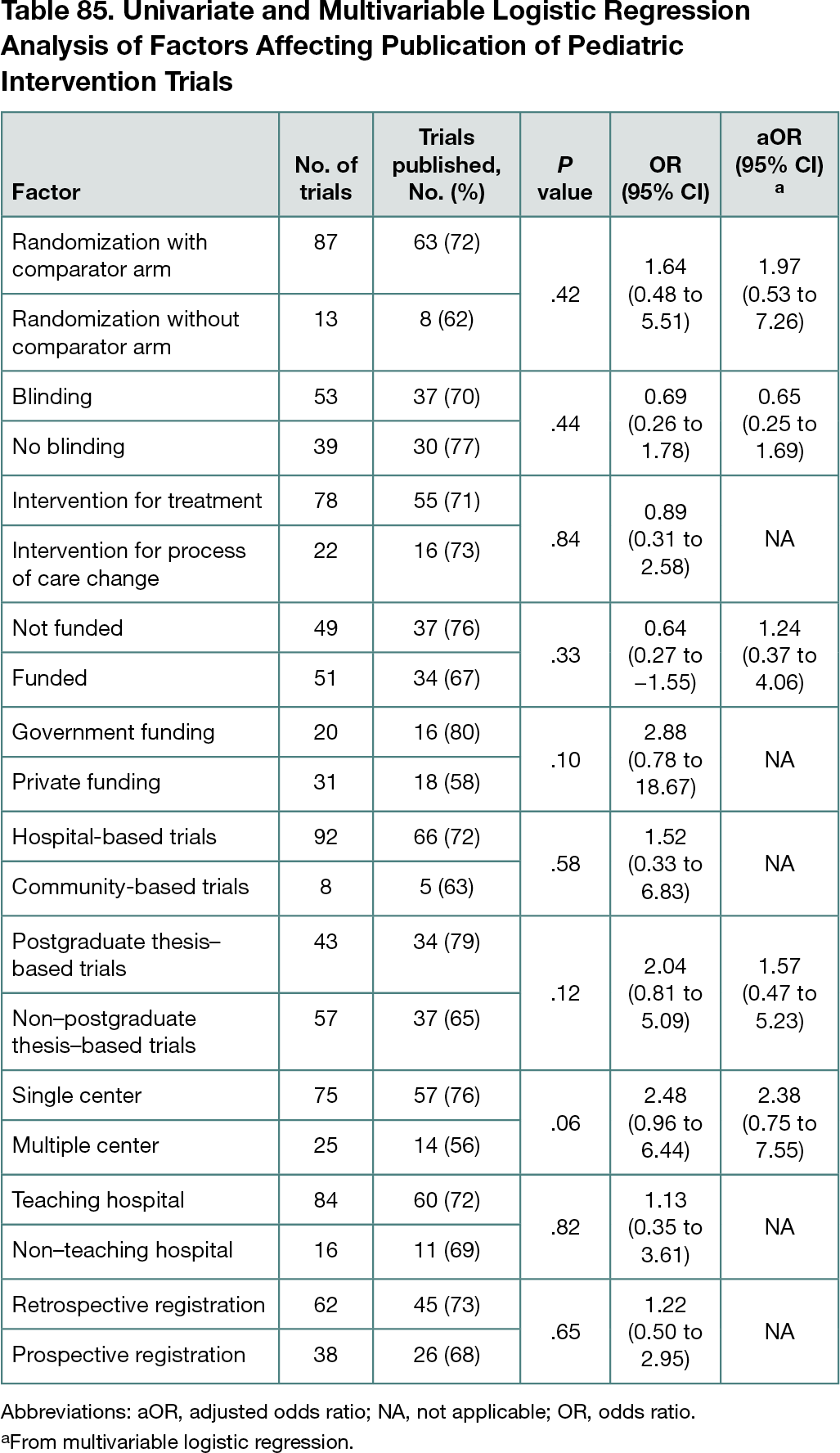
The first 100 pediatric intervention trials registered from 2008 to 2012 were retrieved from the CTRI. The overall proportion of trials published was 71%; 87% had randomization with a comparator arm, 78% examined intervention for treatment, 22% examined intervention for process of care change, 62% had retrospective registration, 51% had funding, and 39% had government funding. The proportion of trials that were performed at a single center was 75%, 92% of trials were hospital based, 84% were conducted at teaching hospitals, and 43% were postgraduate thesis based. The median (range) time to publication was 4 (1-9) years. A non–statistically significant higher proportion of postgraduate thesis–based trials vs non–thesis-based trials (79% vs 65%; OR, 2.04; 95% CI, 0.81-5.09) and single-center trials vs multicenter trials (76% vs 56%; OR, 2.48; 95% CI, 0.96-6.44) were published. On multivariable logistic regression, none of the factors were associated with higher odds of publication (Table 85).
Conclusions
Seventy-one percent of pediatric intervention trials registered in the CTRI were subsequently published regardless of their blinding, funding status, type of intervention, comparator, or setting. A non–statistically significant higher proportion of postgraduate thesis–based trials and single-center trials were published. Further studies with a larger sample size are needed to demonstrate any statistical significance. Journal editors, funding agencies, and ethics committees could implement mandatory registration of intervention trials, as registration seems to be associated with better quality and a good chance of subsequent publication. Following guidelines for preparing data sets for submission to data repositories could help achieve more trial registrations.
References
1. Rao MVV, Maulik M, Juneja A, et al. Clinical Trials Registry—India: a decadal perspective. Indian J Pharmacol. 2020;52(4):272-282. doi:10.4103/ijp.IJP_24_20
2. DePasse JM, Park S, Eltorai AEM, Daniels AH. Factors predicting publication of spinal cord injury trials registered on www.ClinicalTrials.gov. J Back Musculoskelet Rehabil. 2018;31(1):45-48. doi:10.3233/BMR-169628
3. National Heart, Lung, and Blood Institute. Guidelines for preparing clinical study data sets for submission to the NHLBI data repository. Accessed February 20, 2022. https://www.nhlbi.nih.gov/grants-and-training/policies-and-guidelines/guidelines-for-preparing-clinical-study-data-sets-for-submission-to-the-nhlbi-data-repository
1Department of Pediatrics, University College of Medical Sciences, New Delhi, India,2Department of Pediatrics, Maulana Azad Medical College, New Delhi, India
Conflict of Interest Disclosures
None reported.