Abstract
Enrollment and Representativeness in Contemporary Asthma Clinical Trials
Leslie L. Chang,1,2 Clement D. Lee,1,3 Katherine S. Takvorian1,2
Objective
Asthma disproportionately affects historically marginalized racial and ethnic populations in the US.1 However, these populations have been reported to be underrepresented in clinical trials,2 limiting generalizability of study conclusions. The aim of this study was to examine enrollment and representativeness of study populations in contemporary asthma clinical trials.
Design
A systematic search of the PubMed database was performed to identify randomized clinical trials enrolling 100 or more individuals aged 18 years or older with asthma living in the US that were published between January 1, 2015, and December 31, 2021. Exclusion criteria included trials in which asthma was not the major disease, international trials, and secondary analyses or meta-analyses of primary trials. Qualifying trials were abstracted for basic trial information, study design, and baseline epidemiological characteristics of the participants. To evaluate representativeness of study populations compared with Centers for Disease Control and Prevention epidemiological data for people with asthma, standardized mean differences using Cohen d effect size were calculated; underrepresentation and overrepresentation were defined as the proportions of enrolled participants that were 1 or less SD and 1 or more SD of national proportions, respectively.
Results
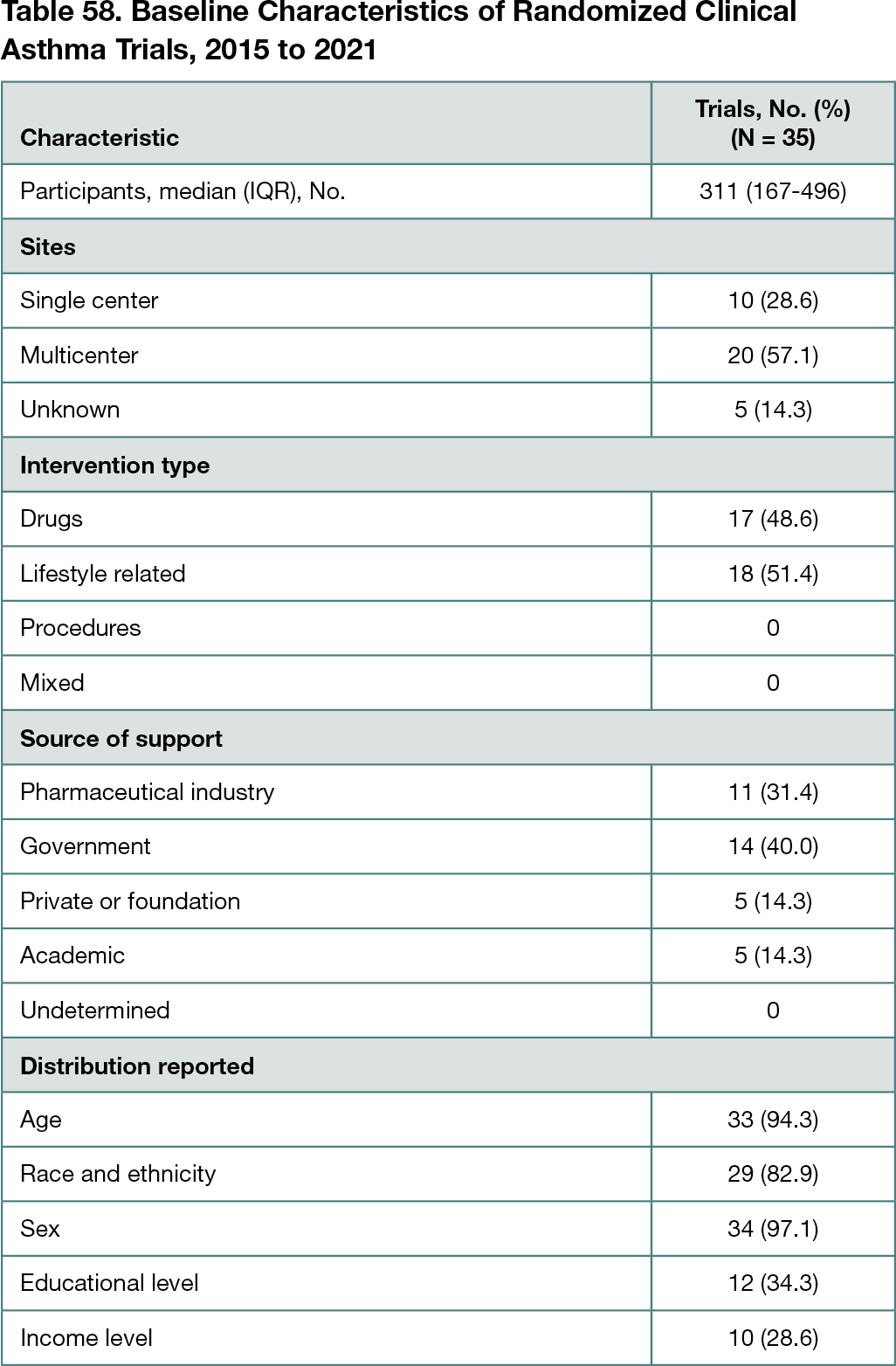
Of the 1032 trials screened, 35 met inclusion criteria (with the majority excluded due to international enrollment). The trials included a median of 311 participants (IQR, 167-496 participants). Approximately half of the trials were drug intervention trials, and the remainder investigated educational or behavioral interventions. There was a mix of funding sources, including pharmaceutical, government, academic, and private institutions. Almost all of the trials reported age and sex distribution of the participants. A total of 29 trials (82.9%) reported race or ethnicity. Only 12 (34.3%) and 10 (28.6%) trials reported educational and income levels, respectively (Table 58). With respect to representativeness of the study populations, among trials reporting participant sex, 1 of 33 (3.0%) had a proportion of females that was overrepresentative of the US population with asthma and no trials were underrepresentative. Among trials reporting Black race, 5 of 25 (20.0%) enrolled a proportion of Black participants that was overrepresentative of the US population with asthma and no trials were underrepresentative. Among trials reporting Hispanic ethnicity, 1 of 16 (6.25%) was overrepresentative and no trials were underrepresentative. Among trials reporting White race, 4 of 27 (14.8%) enrolled proportions that were underrepresentative of the US population with asthma and no trials were overrepresentative.
Conclusions
In contemporary clinical trials of adults with asthma in the US, the majority of trials reported participants’ age, sex, and race and fewer reported education and income information. The study did not find that historically marginalized groups were systematically underrepresented in asthma clinical trials. Collecting and reporting patients’ demographic and socioeconomic characteristics is crucial to understanding and generalizing trial results.
References
1. Asthma and Allergy Foundation of America. Asthma disparities in America. Accessed February 25, 2022. https://www.aafa.org/asthma-disparities-burden-on-minorities.aspx
2. Downing NS, Shah ND, Neiman JH, Aminawung JA, Krumholz HM, Ross JS. Participation of the elderly, women, and minorities in pivotal trials supporting 2011-2013 US Food and Drug Administration approvals. Trials. 2016;17(1):199. doi:10.1186/s13063-016-1322-4
1New England Journal of Medicine, Boston, MA, USA, clee@nejm.org; 2Department of Medicine, Massachusetts General Hospital, Boston, MA, USA; 3Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA
CONFLICT OF INTEREST DISCLOSURES
Leslie L. Chang, Clement D. Lee, and Katherine S. Takvorian are editorial fellows at the New England Journal of Medicine.