Abstract
Dissemination of the Results of Pediatric Clinical Trials Funded by the US National Institutes of Health
Chris A. Rees,1 Adrianna Westbrook,2 Florence T. Bourgeois3
Objective
The National Institutes of Health (NIH) is the largest source of government funding for biomedical research in the world, although only a small proportion of its funding is dedicated to pediatric research. Timely dissemination of results for NIH-funded pediatric clinical trials is imperative to uphold the integrity of scientific evidence, maintain ethical obligations to trial participants, and ensure evidence-based clinical care. Since 2017, the NIH has required NIH-funded clinical trials to be registered and summary results submitted to ClinicalTrials.gov, generally not later than 1 year after primary trial completion.1 In this study, the dissemination of NIH-funded pediatric clinical trial findings was investigated through results submitted to ClinicalTrials.gov and in publications in peer-reviewed medical journals.
Design
A cross-sectional analysis of NIH grants funding pediatric clinical trials with funding completed from January 1, 2017, to December 31, 2019, was conducted. Grants funding pediatric trials in NIH RePORTER and the status of registration and results submission in ClinicalTrials.gov were determined. Publications were identified in PubMed as of February 28, 2022. Time to results reporting in ClinicalTrials.gov at 12 months and 24 months since primary trial completion, and factors associated with nonpublication from an a priori set of variables using multivariable logistic regression were determined.
Results
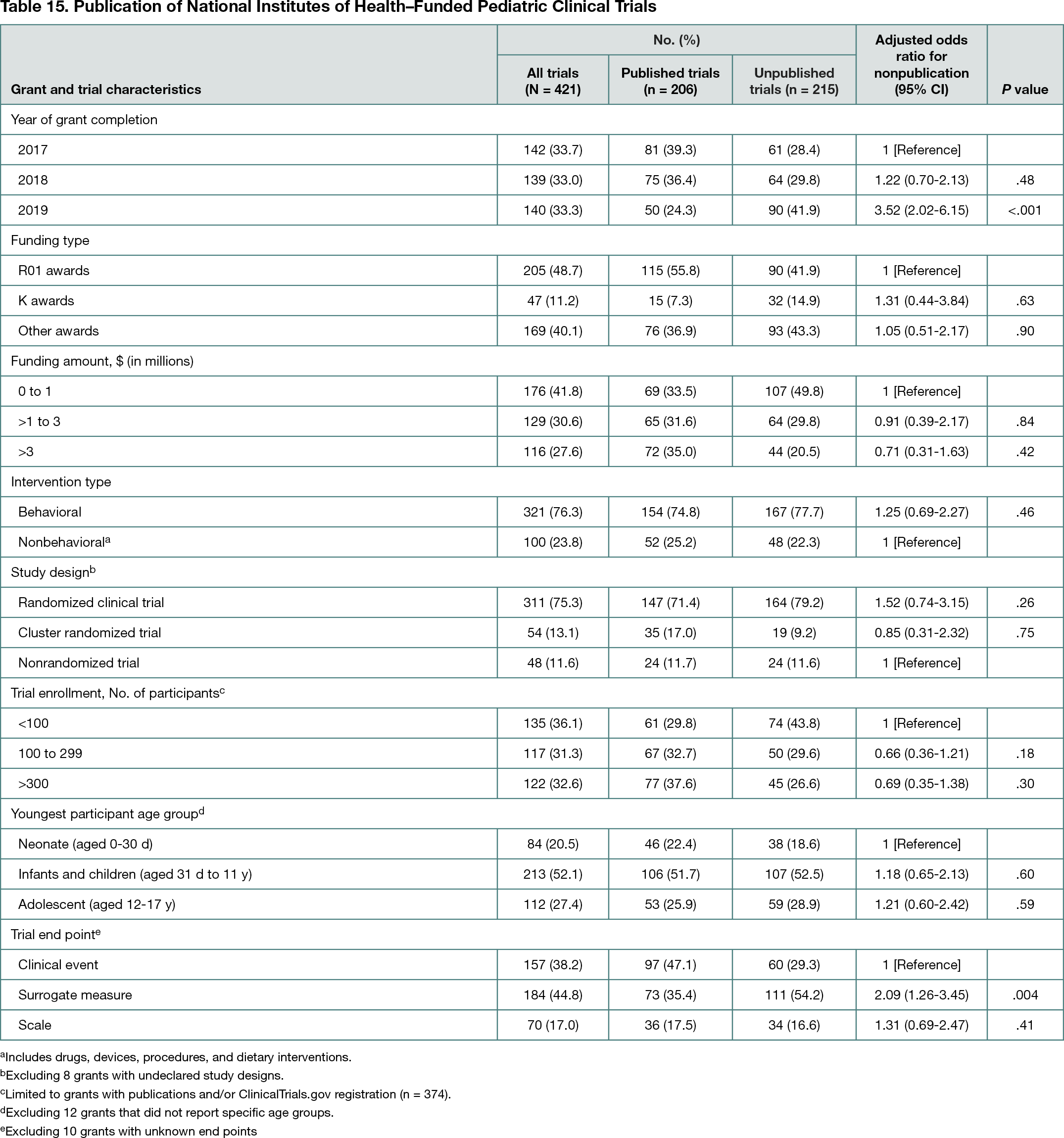
Among 3408 pediatric grants completed during the study period, 421 (12.4%) supported an interventional study (Table 15). Mental and behavioral health conditions (70 trials [16.6%]), obesity (56 [13.3%]), and substance use (40 [9.5%]) were the most commonly studied conditions. Greater than three-quarters of the trials studied behavioral interventions (321 [76.2%]). There were 360 trials (85.5%) registered in ClincialTrials.gov, of which 229 (63.6%) were registered prospectively (ie, within 21 days of study start). Results were submitted for 16.6% of trials (95% CI, 14.4%-18.8%) by 12 months and 22.8% (95% CI, 20.2%-25.5%) by 24 months. Publications were available for 11.8% of trials (95% CI, 10.2%-13.5%) at 12 months and 27.7% (95% CI, 25.3%-30.1%) at 24 months. Overall, 56,833 participants were enrolled in trials that remained unpublished after a median follow-up of 33 months since trial completion. Trials were more likely to remain unpublished if the trial end point was a surrogate measure (adjusted odds ratio, 2.09; 95% CI, 1.26-3.45) compared with a clinical event.
Conclusions
Despite policies promoting dissemination of NIH-funded clinical trial results, less than a third of pediatric trials reported results in ClinicalTrials.gov or published findings in peer-reviewed journals 2 years after completion. To maximize the impact of pediatric clinical trials, additional efforts are needed to improve reporting practices and advance translation of research findings into evidence-based clinical care. Such efforts may include the withholding of additional funding unless trials have been prospectively registered and results publicly reported in a timely manner.
Reference
1. NIH policy on the dissemination of NIH-funded clinical trial information. National Institutes of Health. Updated July 24, 2017. Accessed March 5, 2022. https://grants.nih.gov/policy/clinical-trials/reporting/understanding/nih-policy.htm
1Division of Pediatric Emergency Medicine, Emory University School of Medicine, Children’s Healthcare of Atlanta, Atlanta, GA, USA, chris.rees@emory.edu; 2Pediatric Biostatistics Core, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA; 3Pediatric Therapeutics and Regulatory Science Initiative, Computational Health Informatics Program, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
Conflict of Interest Disclosures
No authors have potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this abstract.