Abstract
Development of the Standards for Reporting Subtyping Studies (StaRSS) Reporting Guideline
Seyed-Mohammad Fereshtehnejad,1,2 Connie Marras,3,4 David Moher,5,6 Tiago Mestre,1 for the International Parkinson and Movement Disorder Society Task Force on Parkinson’s Disease’s Subtypes
Objective
In the evolving era of precision medicine, a subtyping study attempts to define subgroups of a disease entity wherein certain individuals with the disease share clinical characteristics (eg, biomarker profile, clinical manifestations, and prognosis) distinct from others with the disease. There is an increase in the number of studies on disease subtyping in the past 40 years, with a rapid surge in the last decade, yet there is no standardized reporting guideline or appraisal checklist for subtyping studies to promote reporting quality. This new guideline aimed to improve the accuracy and completeness of reporting of studies of disease subtyping and to aid readers and peer reviewers in assessing the potential for bias in a subtyping study.
Design
Members of the International Parkinson and Movement Disorder Society Task Force on Parkinson’s Disease’s Subtypes, having performed a systematic review of subtyping studies of Parkinson disease and found significant methodologic shortcomings of published subtyping research,1 followed up with a systematic search of the Enhancing the Quality and Transparency of Health Research (EQUATOR) Network database and others to find published guidelines about subtyping research, with no results.2 In response, the Task Force convened expert panel meetings to develop a Standards for Reporting Subtyping Studies (StaRSS) reporting guideline,1 following the steps introduced in the guidance for developers of health research reporting guidelines.3 To do so, members of the expert panel created new items relevant to the context of subtyping using Parkinson disease as a model. The items were approved after multiple revisions in expert panel meetings.
Results
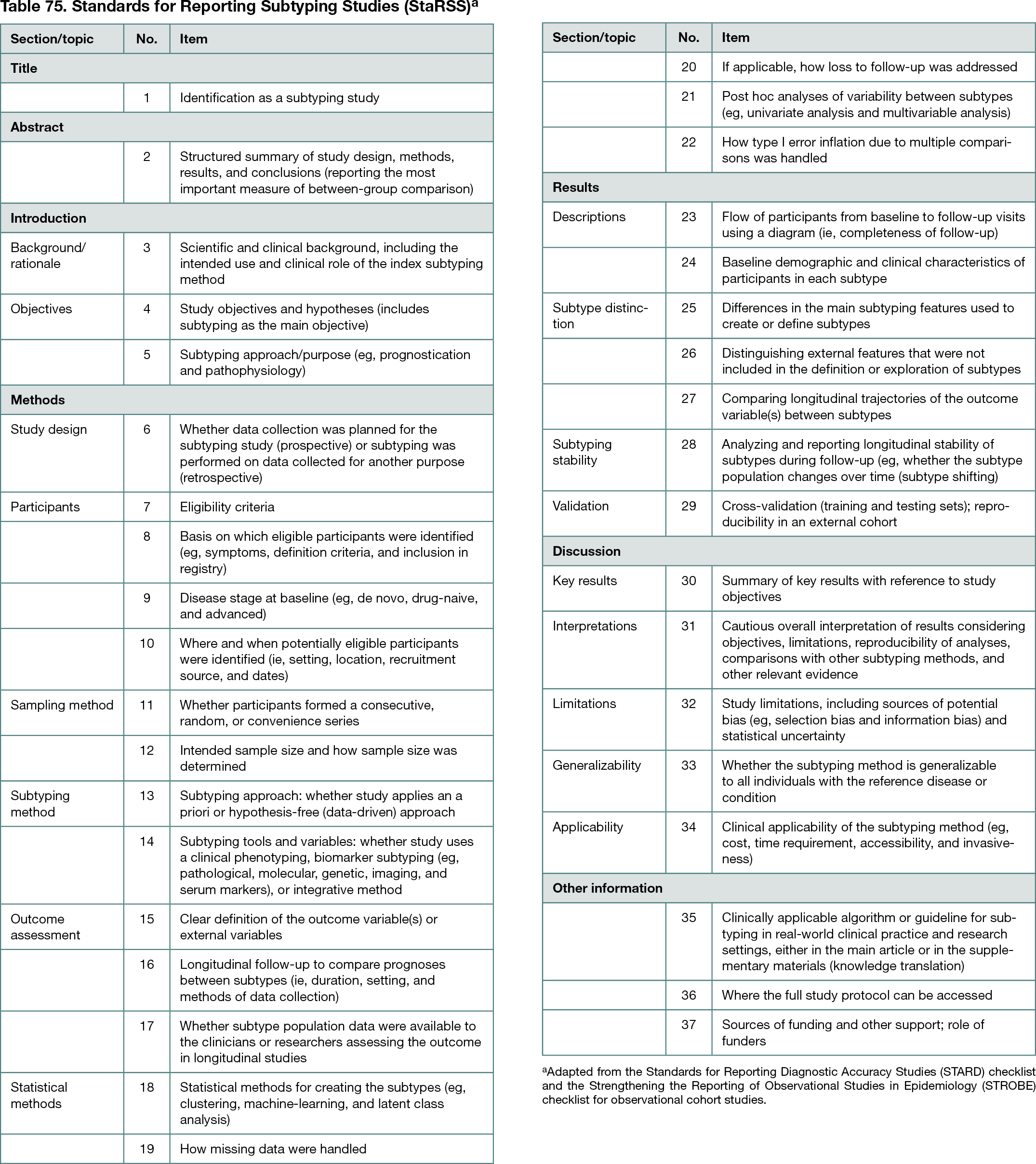
The reporting guideline comprises 37 items, including numerous novel topics unique to the concepts and various aspects of a subtyping study mainly related to the Methods and Results sections of subtyping studies (Table 75). For the other sections, the Task Force adopted relevant items from existing general guidelines, namely, the Standards for Reporting of Diagnostic Accuracy (STARD) and the Reporting of Observational Studies in Epidemiology (STROBE), with minor adjustments.
Conclusions
StaRSS is a consensus guideline that describes essential elements of the design, outcome assessment, execution of statistical tests, and reporting of subtype research findings and could guide authors to properly conduct a subtyping study. Adoption of the StaRSS checklist by biomedical journals might improve the quality of reporting and appraisal of subtyping studies by peer reviewers. To achieve these goals, future studies are needed to assess the utility of the StaRSS checklist through panel discussions of the experts in subtyping studies of various fields of biomedicine.
References
1. Mestre TA, Fereshtehnejad SM, Berg D, et al. Parkinson’s disease subtypes: critical appraisal and recommendations. J Parkinsons Dis. 2021;11(2):395-404. doi:10.3233/JPD-202472
2. Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: reporting guidelines for health research. Lancet. 2008;371(9619):1149-1150. doi:10.1016/S0140-6736(08)60505-X
3. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2):e1000217. doi:10.1371/journal.pmed.1000217
1Division of Neurology, Department of Medicine, University of Ottawa, Ottawa, ON, Canada, sm.fereshtehnejad@ki.se; 2Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society (NVS), Karolinska Institutet, Stockholm, Sweden; 3Movement Disorders Clinic, Toronto Western Hospital, University Health Network, Toronto, ON, Canada; 4Division of Neurology, Department of Medicine, University of Toronto, Toronto, ON, Canada; 5Ottawa Methods Centre, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada; 6Department of Epidemiology and Community Medicine, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
Conflict of Interest Disclosures
Seyed-Mohammad Fereshtehnejad received honoraria from the European Science Foundation. Connie Marras is a consultant for Grey Matter Technologies and receives financial compensation as a steering committee member from the Michael J. Fox Foundation and the Parkinson’s Foundation (USA); she received research grants from the Michael J. Fox Foundation, Canadian Institutes of Health Research, International Parkinson and Movement Disorder Society, and the Weston Brain Institute and as a site investigator for clinical studies sponsored by Theravance Inc, the Michael J. Fox Foundation, the Parkinson’s Foundation, and Centogene. David Moher is an associate director of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract. Tiago Mestre reports speaker honoraria from AbbVie and the International Parkinson and Movement Disorder Society; consultancies from CHDI Foundation/management and advisory board from AbbVie; Biogen, Roche, nQ Medical, Medtronic, Sunovion, and Valeo Pharma; and research funding from EU Joint Programme–Neurodegenerative Disease Research, University of Ottawa Brain and Mind Research Institute, Ontario Research Fund, Canadian Institutes of Health Research, the Michael J. Fox Foundation, Parkinson Canada, Parkinson’s Disease Foundation/Parkinson Study Group, LesLois Foundation, PSI Foundation, and the Parkinson Research Consortium.
Acknowledgments
We would like to thank all the members of the Parkinson’s disease subtyping task force at the International Movement Disorder Society.