Abstract
Development of the Accurate Consensus Reporting Document (ACCORD) Reporting Guideline
Patricia Logullo,1 Esther J. van Zuuren,2 A. Pali S. Hungin,3 Christopher C. Winchester,4 David Tovey,5 Ellen L. Hughes,6 Keith H. Goldman,7 Niall Harrison,8 William Gattrell9
Objective
Consensus methodologies are widely used to harness expert knowledge for decision-making in areas of uncertainty. While specific guidance is available on conducting and reporting Delphi initiatives in palliative care,1 there remains a need for broader reporting guidelines. The ACCORD (Accurate Consensus Reporting Document2) initiative will develop guidance for transparent and complete reporting of consensus-building methodologies in biomedical research and clinical practice. This abstract reports findings from a systematic literature review that will inform consensus on checklist items for ACCORD.
Design
Studies, reviews, and guidance documents addressing the quality of reporting of consensus methods in biomedicine or clinical practice were eligible for inclusion. Reports of consensus methods that did not comment on reporting quality were excluded. Searches were conducted with no limits by year or language. Identified articles were retrieved and assessed for eligibility using Rayyan by 4 evaluators working independently; discrepancies were reconciled by discussion. The search process started on January 7, 2022; the assessment period was completed on February 7, 2022. Data extraction was done using Covidence, and potential checklist items were generated.
Results
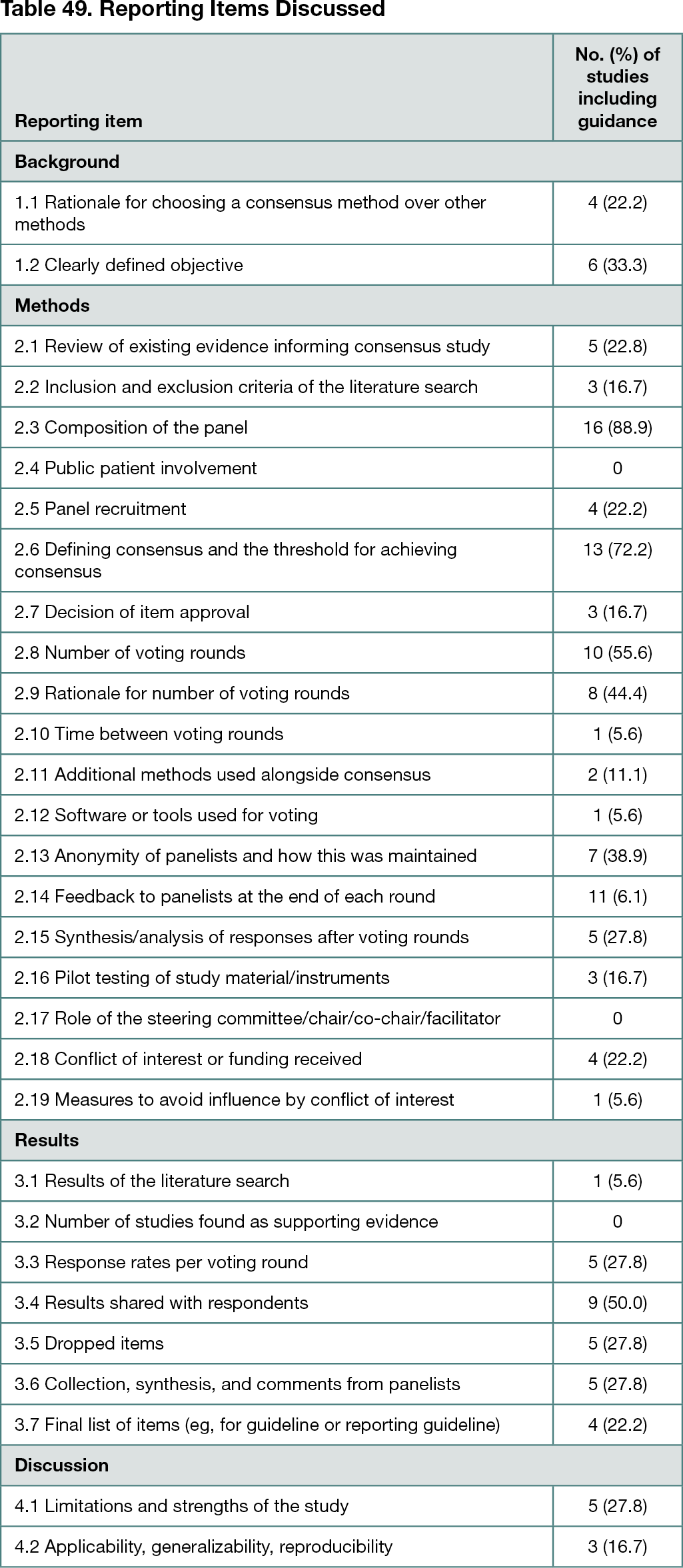
Overall, 2736 references were identified: 2599 articles and documents (Web of Science, 1775; MEDLINE [Web of Science], 1501 [202 unique]; PubMed, 375 [219 unique]; MEDLINE [OVID], 641 [174 unique]; Embase, 331 [66 unique]; Cochrane, 131 [77 unique]; Emcare, 179 [29 unique]; Academic Search Premier, 280 [23 unique]; and PsycINFO, 173 [34 unique]) and 137 meeting abstracts (Web of Science, 14; Embase, 99 [90 unique]; Cochrane, 36 [33 unique]). In all, 54 publications were selected for full-text review; 18 met the eligibility criteria. Most studies acknowledged that reporting quality of consensus initiatives could be improved. The most discussed items included panel composition and consensus definition and thresholds (Table 49). Public and patient involvement and roles of the steering committee and chair(s) were among the least addressed.
Conclusions
Most identified studies acknowledged the need to improve reporting quality of consensus methodologies. ACCORD aims to set a standard for reporting consensus initiatives, improving their transparency and making it easier to critically appraise the methods used to develop consensus recommendations.
References
1. Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on conducting and reporting delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684-706. doi:10.1177/0269216317690685
2. Gattrell WT, Hungin AP, Winchester CC, et al. ACCORD guideline for reporting consensus-based methods in biomedical research and clinical practice: a study protocol. Res Integr Peer Rev. 2022;7(3): doi:10.1186/s41073-022-00122-0
1Centre for Statistics in Medicine (CSM), University of Oxford, and EQUATOR Network UK Centre, Oxford, UK, patricia.logullo@ndorms.ox.ac.uk; 2Leiden University Medical Centre, Leiden, Netherlands; 3University of Newcastle, Newcastle upon Tyne, UK; 4Oxford PharmaGenesis, Oxford, UK; 5Journal of Clinical Epidemiology, London, UK; 6Sciwright Limited, Somerset, UK; 7AbbVie, North Chicago, IL, USA; 8Ogilvy Health, London, UK; 9Ipsen, Milton Park, UK
Conflict of Interest Disclosures
Patricia Logullo is a member of the UK EQUATOR Centre, Oxford, UK, an organization that promotes the use of reporting guidelines, many of which are developed using consensus methods, and she is personally involved in the development of other reporting guidelines. A. Pali S. Hungin worked with Reckitt Benckiser in the last 5 years for the development of the definitions and management of gastroesophageal reflux disease. Christophe C. Winchester is an employee, director, and shareholder of Oxford PharmaGenesis, Oxford, UK; a director of Oxford Health Policy Forum CIC; a trustee of the Friends of the National Library of Medicine; and an associate fellow of Green Templeton College. Ellen L. Hughes has worked with Ogilvy Health UK on consensus projects. William Gattrell was an employee of Ipsen, Oxford, UK, at the time of the analysis. He is now an employee of Bristol Myers Squibb. No other disclosures were reported. No authors were reimbursed for participating in the initiative.