Abstract
Development and Testing of a Tool to Assist Editorial Staff in Review of Ethical Research Reporting in Manuscripts
Jan Higgins,1 Robert D. Steiner,2 Katharine Murphy,1 Kyle Brothers3
Objective
Journals are obligated to ensure published research is conducted ethically, with this task often falling to editorial staff. However, editorial staff are rarely trained in research ethics.
Design
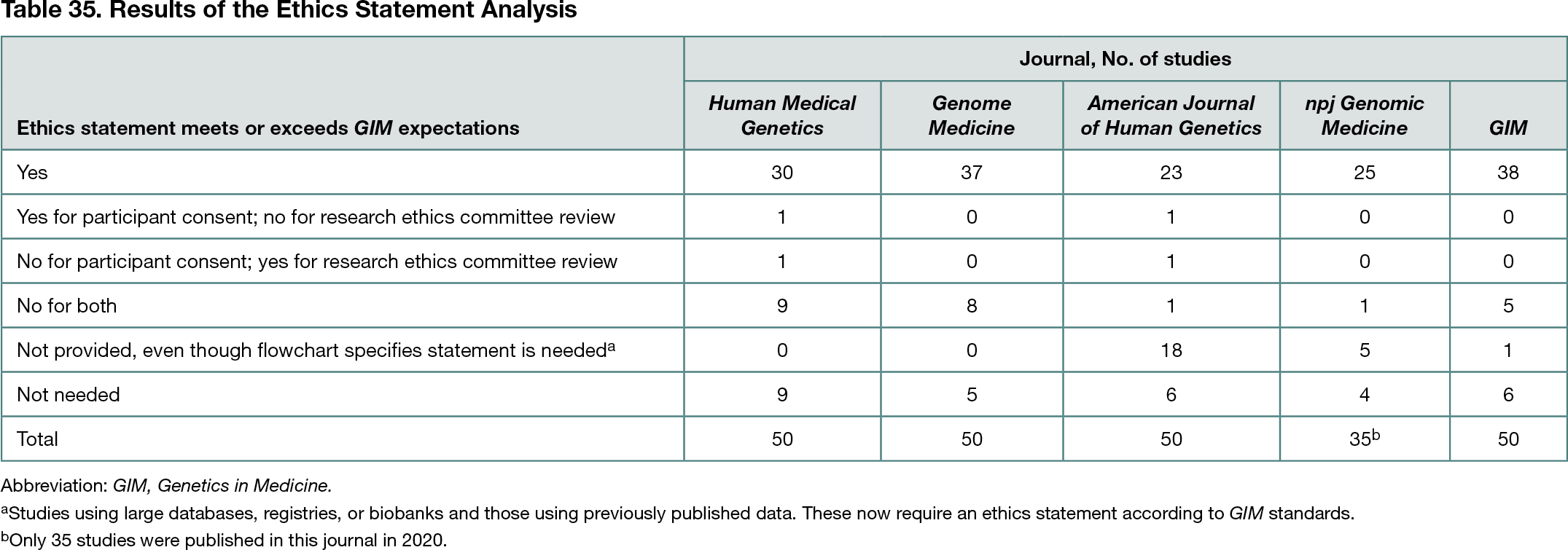
Genetics in Medicine (GIM) staff and editors developed a flowchart as a tool to categorize research studies submitted for publication. Twelve categories were defined (human participants or individual-level data; human biosamples/biobanks; transplantation; newborn screening blood spots; previously published data; database data; surveys; animal studies; electronic health records; quality assurance/quality control/operations; algorithm/software development; and deceased patient samples.) The GIM Ethics Advisory Committee (EAC) determined ethics statements required for publication. To test this tool, 50 consecutive articles from GIM prior to implementing the tool (2020) and 50 consecutive articles from 4 peer journals (Human Medical Genetics, Genome Medicine, American Journal of Human Genetics, and npj Genomic Medicine [all in 2020]) were analyzed. For each article, the following were assessed: (1) whether the flowchart provided appropriate categories; (2) whether an ethics statement was provided; and (3) whether the ethics statement met the standards defined by the GIM EAC.
Results
Review of articles from GIM prior to implementation of the tool and from 4 peer journals revealed that the majority comply with ethical standards (Table 35). A small number of articles lacked one or both elements of the ethics statement. These studies might have been conducted without appropriate permissions, or the journal might not have required a statement. The latter seems the most likely explanation for research using large databases, registries, or biobanks, as well as for studies based on previously published data. GIM now requires ethics statements for these categories, but other journals appear not to share this requirement.
Conclusions
This tool facilitated review of articles by editorial office staff to ensure reporting of ethical conduct of research prior to publication and facilitates auditing for compliance with ethical standards.
1American College of Medical Genetics and Genomics, Bethesda, MD, USA, gim@acmg.net; 2Marshfield Clinic Health System and University of Wisconsin School of Medicine and Public Health, Marshfield and Madison, WI, USA; 3University of Louisville and Norton Children’s Research Institute, Louisville, KY, USA
Conflict of Interest Disclosures
Robert D. Steiner reports employment with PreventionGenetics/Exact Sciences; consulting and equity interest with Acer Therapeutics and PTC Therapeutics; consulting with Aeglea, Health Advances, Leadiant, Precision for Value, and Travere; honoraria from Medscape/WebMD and Teladoc; and research support from Alexion and the Smith Lemli Opitz Syndrome Foundation.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the American College of Medical Genetics and Genomics.