Conflict of Interest Network Robustness and Funder Homogeneity Associated With Reported Adverse Events and Deaths in Published Drug Studies
Abstract
S. Scott Graham,1 Joshua B. Barbour,2 Zoltan P. Majdik,3 Madeline Bruegger,1 Carlee A. Baker,1 Justin F. Rousseau4
Objective
Research on industry funding and author conflicts of interest (COIs) suggests that centralization of funding may lead to detrimental effects on biomedical research.1,2 This study was conducted to assess relationships between COI networks and drug adverse event rates reported to the US Food and Drug Administration.
Design
We used a GPT 4.0 pipeline (a series of computational steps applied to a dataset for transformation and/or analysis) to identify and classify reported author COIs and funders associated with 302 of the most commonly used drugs in the US. Included articles included up to 150 of the most relevant PubMed results when searching for each drug’s proprietary and generic name. For each declared COI, the funder was identified and funder types were classified by a fine-tuned GPT 4.0 model as industry, federal, university, patient advocacy organization, professional society, university, or hospital/clinic. COI relationships for each drug product were assembled into network models mapping relationships among all reported funders and all articles for each product. Each network was evaluated for network robustness by measuring critical failure threshold (CFT) (ie, the minimum number of nodes that would need to be removed to result in a 50% collapse of the network).3 COI network robustness is a useful measure of the centralization of the funding network as a low number of funder networks would have lower failure thresholds. A network with many independent funders would require more nodes to be removed before network failure. Controlling for COI rates, drug features (over-the-counter availability, generic availability, US Drug Enforcement Administration schedule, usage rates), and publication features (mean article year of publication, total authorships in the network), we evaluated the relationship between network robustness and reported adverse events rates (total, serious, and fatal). A secondary model was fit to determine whether individual funder type frequencies or heterogeneity were associated with failure threshold. The GPT classifier was compared with a human annotated sample and evaluated for precent accuracy.
Results
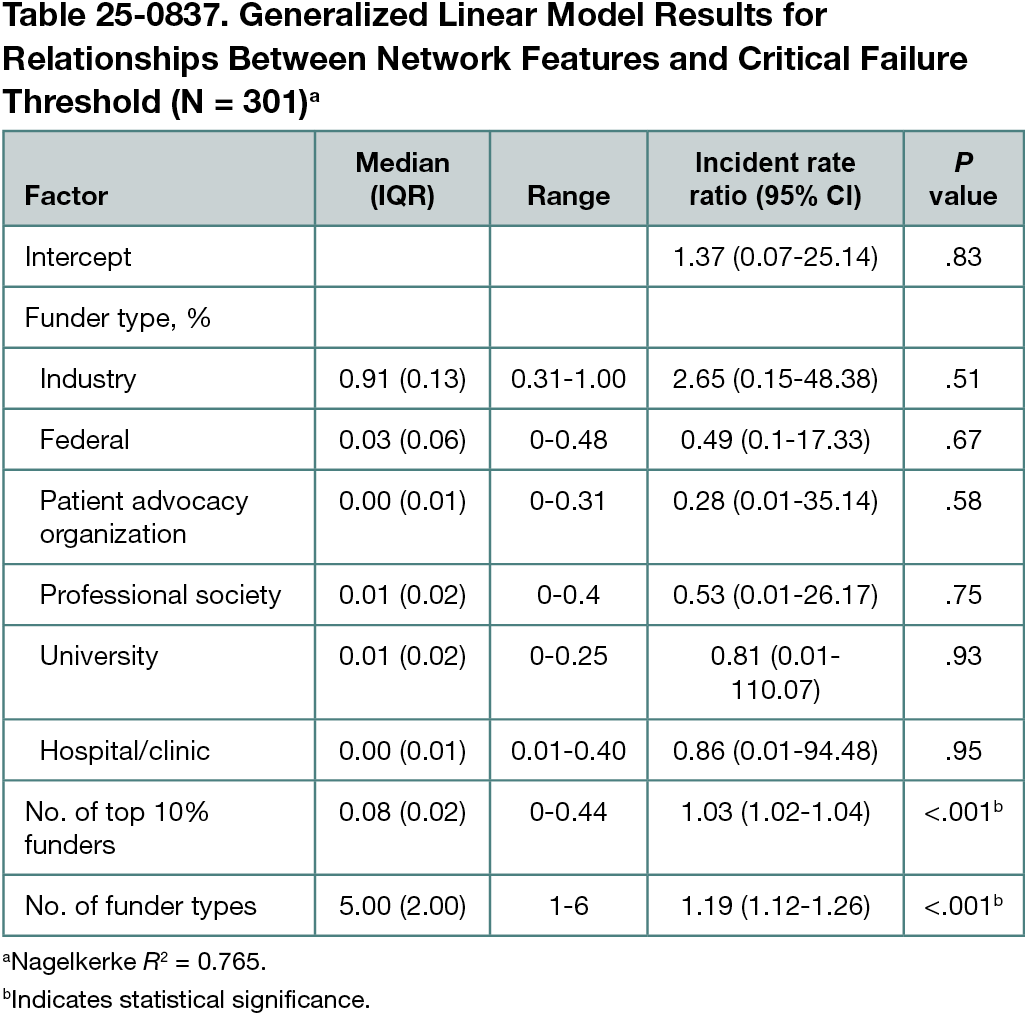
We identified 37,993 articles associated with the 302 drugs. The GPT 4.0 classifier achieved 98.9% accuracy compared with the human annotated sample. In the adverse events models, CFT returned adjusted incident rate ratios (IRRs) of 0.94 (95% CI, 0.91-0.97; P < .01) for total events, 0.94 (95% CI, 0.91-0.97, P < .01) for serious events, and 0.94 (95% CI, 0.91-0.98; P < .01) for fatal events. The number of funders per network was not a significant factor in any adverse events model. The network features model (Table 25-0837) returned significant relationships between the CFT and the number of funders in the top 10% by out-degree, a network science measure of the number of outgoing connections from a given node (IRR, 1.03 [95% CI, 1.02-1.04]; P < .001) and funder-type heterogeneity (IRR, 1.19 [95% CI, 1.12-1.26]; P < .001).
Conclusions
In this study, COI network robustness was inversely associated with adverse events, including serious and fatal events, reported in drug studies. Secondary analyses indicate that this was associated with funding network centralization and homogeneity. The products evaluated in this study were associated with lower adverse event rates when the collected research had a larger number of funders, regardless of funder type. This suggests that research funding policy should encourage funding diversity.
References
1. Graham SS, Harrison KR, Edward JC, Majdik ZP, Barbour JB, Rousseau JF. Beyond bias: aggregate approaches to conflicts of interest research and policy in biomedical research. World Med Health Policy. 2024;16(3):489-505. doi:10.1002/wmh3.608.
2. Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4(9):e286. doi:10.1371/journal.pmed.0040286
3. Albert R, Jeong H, Barabási AL. Error and attack tolerance of complex networks. Nature. 2000;406(6794):378-382. doi:10.1038/35019019
1Department of Rhetoric & Writing, The University of Texas at Austin, Austin, TX, US, ssg@utexas.edu; 2Department of Communication, University of Illinois Urbana-Champaign, Urbana, IL, US; 3Department of Communication, North Dakota State University, Fargo, ND, US; 4Department of Neurology & Peter O’Donnell Jr. Brain Institute, The University of Texas Southwestern Medical Center, Dallas, TX, US.
Conflict of Interest Disclosures
S. Scott Graham received grant funding from the National Institute of General Medical Sciences (NIGMS). Joshua B. Barbour received grant funding from the NIGMS, NSF, IC2, and Blue Cross Blue Shield; royalties form Cengage Publishing; and consulting fees from The Aslan Group and BrainCheck. Justin F. Rousseau received grant funding from the NIGMS, National Library of Medicine, National Institute on Aging, National Institute of Allergy and Infectious Diseases, National Institute of Mental Health, National Center for Advancing Translational Sciences, Texas Child Mental Health Care Consortium, Michal & Susan Dell Foundation, Texas Alzheimer’s Research and Care Consortium, Austin Public Health, and the Health Care Cost Institute. No other disclosures were reported.
Funding/Support
Funding for this research was provided by the NIGMS of the National Institutes of Health under award number R01GM141476.
Role of the Funder/Sponsor
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the abstract; and decision to submit the abstract for presentation.
