Assessment of Use of Dedicated Editors for Handling and Reviewing Manuscripts With Previously Obtained Peer Reviews
Abstract
Riaz Qureshi,1 Kirsty Loudon,2 Alexander Gough,3 Shaun Treweek,4 Tianjing Li1
Objective
The objective of this research was to assess whether an editorial workflow wherein submissions are handled solely by dedicated protocol editors reduces the average time from initial submission to a final decision compared with standard peer review and whether this reduction differs by protocol formatting (structured or unstructured).
Design
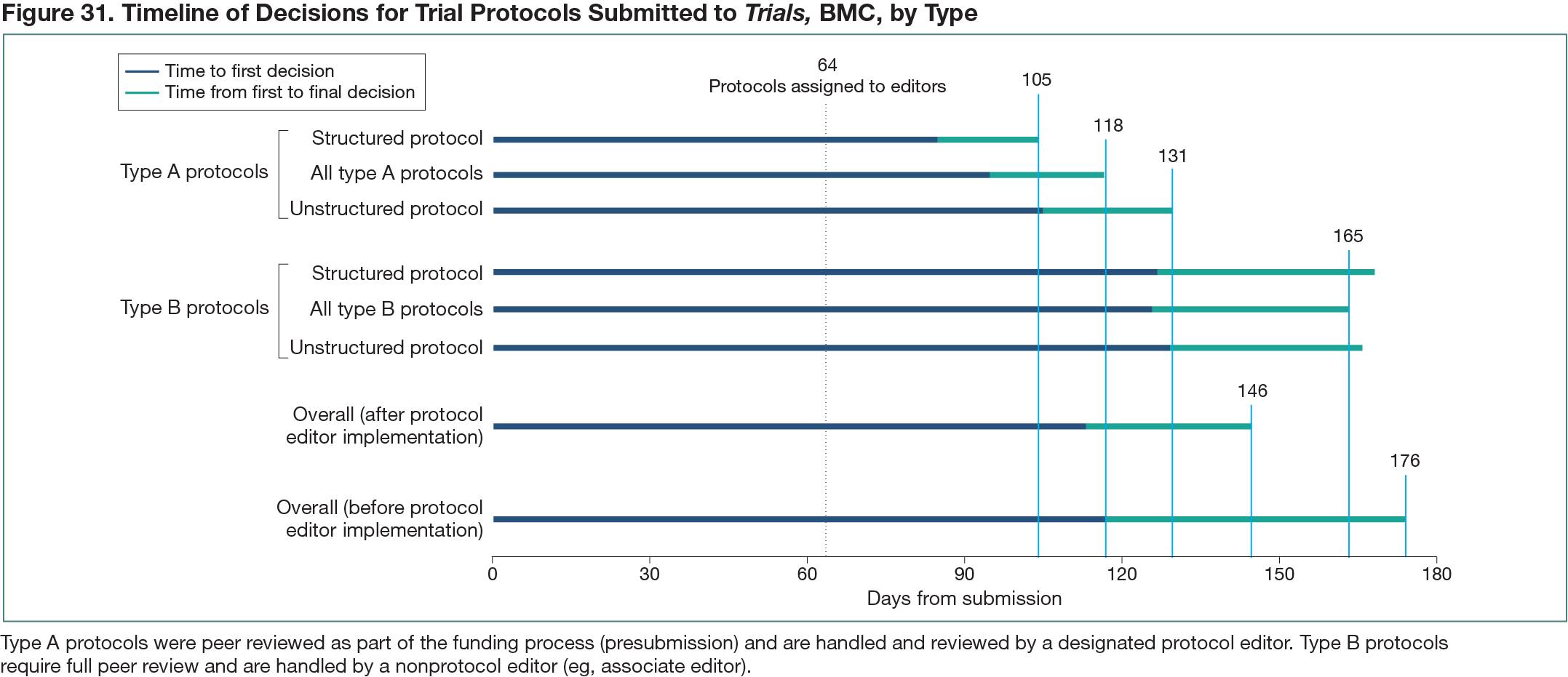
A cross-sectional study of the workflow timing for protocol submissions to Trials was conducted. Type A protocols demonstrate prior peer review as part of their funding process by providing previous comments and self-declaring this prior review in the submission process. Type A protocols are handled by protocol editors as the sole reviewers, whereas type B protocols are handled by other editors and require full peer review (ie, 2 to 3 reviewers). The workflow data stored in the Trials manuscript submission and review system were extracted to compare the timing from initial submission to first and final decisions for all protocols, separated by type, before and after protocol editors were implemented (January to December 2019 and January 2020 to November 2021, respectively). The timing was also extracted for submissions that were formatted as unstructured protocols with an accompanying SPIRIT checklist or following the Structured Protocol Template recommended by Trials. The workflow timing results are descriptively summarized.
Results
From January 2020 to November 2021, 1114 type A (360 [32%]) and type B (754 [68%]) trial protocols were submitted to Trials. Compared with type B protocols, 143 of which (19%) used a structured template, 137 type A protocols (38%) followed a structured format, possibly owing to self-selection or prior reviews leading to a more structured approach in submission. A timeline of the workflow for each protocol type by format as well as overall for each period is shown in Figure 31. Overall, type A protocols had a mean of 118 days to final decision (from initial submission), whereas type B protocols had a mean of 165 days to final decision. Overall, across protocols in the period after protocol editors were implemented, the time to final decision was reduced 30 days from the preceding year: 146 days (January 2020 to November 2021) versus 176 days (January to December 2019). Among type A protocols, a structured template format reduced the time to final decision compared with unstructured—an association that was not seen in type B protocols, possibly because multiple reviewers were required.
Conclusions
The use of dedicated editors to handle trial protocols that had already undergone peer review was associated with fewer days to final decision compared with protocols that were handled by other editors and required full peer review. Future work should examine the quality of peer review in both workflows to determine whether use of dedicated reviewers improves the quality of published works as well as the time to publication.
1Department of Ophthalmology, University of Colorado Anschutz Medical Campus, Aurora, CO, USA, riaz.qureshi@cuanschutz.edu; 2Freelance researcher/editor; 3Institute of Applied Health Research, University of Birmingham, Birmingham, UK; 4Health Services Research Unit, University of Aberdeen, Aberdeen, UK
Conflict of Interest Disclosures
Riaz Qureshi, Kirsty Loudon, and Alexander Gough are protocol editors of Trials, BMC. Shaun Treweek and Tianjing Li are editors in chief of Trials, BMC.
Acknowledgments
We would like to thank Eleanor Cox, Bex Chang, and Krishna Vairamani for their work as editorial staff and for managing the workflow of peer review for Trials.
