Abstract
Assessing the Readability and Quality of Patient or Caregiver Fact Sheets for COVID-19 Therapeutics with Emergency Use Authorization by the Food and Drug Administration
Shelly Melissa Pranić,1 Jasna Karacić2
Objective
Aside from remdesivir, the first US Food and Drug Administration (FDA)–approved drug to treat COVID-19, the FDA has authorized on an emergency basis (known as an emergency use authorization [EUA]) the use of drugs given the relative unavailability of effective COVID-19 treatments.1 This study was conducted to determine fact sheet readability and quality of FDA-approved and EUA nonvaccine drugs or biologicals (therapeutics) to treat COVID-19.
Design
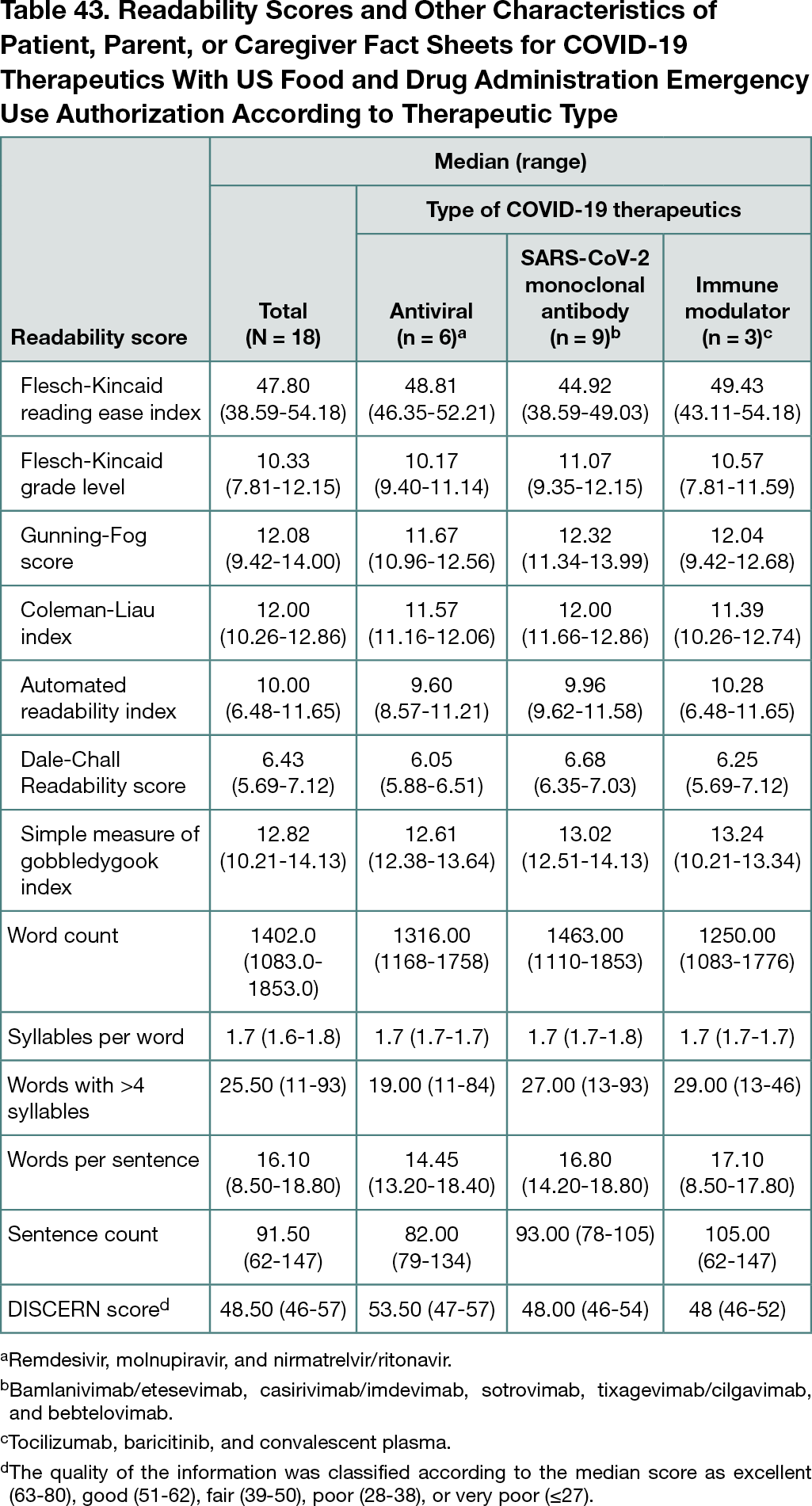
In a cross-sectional study, facts sheets with 1 or more issuances using Google and the term fact sheet and the therapeutic name and FDA EUA website for fact sheets for patients, parents, or caregivers on January 17, 2022, and March 2, 2022, were identified.1 Similarities in quality and readability between fact sheets allowed grouping by therapeutic. Two investigators independently selected eligible English-language fact sheets on FDA-approved drugs and EUAs for COVID-19 treatment. Primary outcomes were readability and quality. Seven readability tests were used including (1) Flesch-Kincaid reading ease (FKRE) index (ranging from 0 to 100 with higher scores corresponding to reading ease); (2) Flesch-Kincaid grade (FKG) level (ranging from grades 0 to 18 [college graduate], with lower grades corresponding to easier readability); (3) Gunning-Fog (GF) score (ranging from grades 0 to 20 [college graduate]); (4) Coleman-Liau index (CLI; ranging from grade 4 to college graduate); (5) automated readability index (ARI; ranging from grades 5 to 22 [college graduate]); (6) New Dale-Chall Readability (NDCR; ranging from grade 4 to college graduate); and (7) simple measure of gobbledygook (SMOG) index (ranging from grade 3 to college graduate). Secondary outcomes were word, syllable, and sentence counts. Agreement between investigators was good (80%) in rating 2 fact sheets for quality using the 16-item DISCERN instrument2 with Likert responses (1 indicates minimum and 5, maximum) where the total is 80 and lowest is 16, corresponding to low-quality information; another investigator rated the remainder. Items on the DISCERN instrument assess the transparency of authorship information and relevance of treatment options to patients as described previously.2
Results
Overall, 18 fact sheets were found that described 6 antiviral (37.5%) (4 for remdesivir and 1 each molnupiravir and nirmatrelvir/ritonavir), 9 SARS-CoV-2–targeting monoclonal antibody (43.8%) (1 for bamlanivimab/etesevimab, 3 for casirivimab/imdevimab, 2 each for sotrovimab, tixagevimab/cilgavimab, and 1 for bebtelovimab), and 3 immune modulator (21.4%) (1 each for tocilizumab, baricitinib, and convalescent plasma) information. Table 43 shows the median FKRE, FKG, GF, CLI, ARI, NDCR, and SMOG reading levels above the sixth grade; quality was fair.
Conclusions
Although of fair quality, the reading grade level of fact sheets intended for patients, parents, or caregivers for COVID-19 therapeutics was high, reflecting a need for FDA officials to enforce readable resources from drug manufacturers.
References
1. US Food and Drug Administration. Emergency use authorization: drugs and non-vaccine biological products. Accessed March 2, 2022. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs
2. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: an instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111. doi:10.1136/jech.53.2.105
1University of Split School of Medicine, Cochrane Croatia, Split, Croatia, shelly.pranic@mefst.hr; 2Croatian Association for the Promotion of Patients’ Rights, Cochrane Croatia, Split, Croatia
Conflict of Interest Disclosures
None reported.