Assessing the Quality and Timeliness of Results Reporting for Clinical Trials on Antimicrobial Agents
Abstract
Megan Curtin,1 Allisun Wiltshire,1 Brix Kowalski,2 Maximilian J. Siebert3
Objective
Antimicrobial resistance (AMR) is one of the leading causes of death globally. Timely and complete reporting of clinical trials involving antimicrobial agents (AMAs) is essential to evaluate the safety and efficacy of potential therapies for public use. This study investigates the quality of results reporting of applicable clinical trials (ACTs) and probable ACTs (pACTs) for AMAs. ACTs are interventional studies (excluding phase 1) regulated by the US Food and Drug Administration with at least one site based in the United States. pACTs adhere to the same criteria; however, these studies were initiated prior to January 18, 2017, when the Final Rule came into effect. The rule strengthened reporting obligations by requiring a designated responsible party for results submission to ClinicalTrials.gov and provided clear ACT designation criteria with stricter compliance requirements. We assess how this regulatory change affected the reporting of trials containing AMAs, focusing on timeliness and study characteristics.
Design
We extracted data from ClinicalTrials.gov for trials involving AMAs with primary completion dates between May 1, 2013, and May 1, 2023. We analyzed the time from primary completion to results reporting and estimated the hazard ratio to compare timeliness between ACTs and pACTs. Additionally, we assessed delays in reporting across different study types and funding sources.
Results
Our search resulted in 2629 trials. We excluded 104 because they only included agents with nonantimicrobial properties. We found 1796 pACTs (71.1%; 95% CI, 69.3%-72.9%) and 756 ACTs (29.9%; 95% CI, 28.2%-31.8%). Among the 2525 trials sampled, 2249 trials (89.1%; 95% CI, 87.8%-90.2%) were reported on ClinicalTrials.gov. There was a median (IQR) of 0.5 years (0.2-1.5) between 1 year after the primary completion date and the results submissions across all studies. The median (IQR) time lag for late reporting for ACTs was 0.3 years (0.1-0.9), while it was 0.6 years (0.2-1.8) for pACTs. Overall, 81.3% (95% CI, 79.7%-82.3%) of trials were reported late (75.0% of ACTs vs 83.6% of pACTs). Our analysis showed that ACTs were more likely to report results earlier than pACTs, with a hazard ratio of 1.4 (95% CI, 1.3-1.5) (Figure 24-0810). Regarding funding sources, trials supported by philanthropic foundations or private donors had the slowest reporting (median [IQR] delay, 0.7 [0.3-1.5] years), whereas government-funded trials had the most timely reporting (median [IQR] delay, 0.4 [0.2-1.0] years).
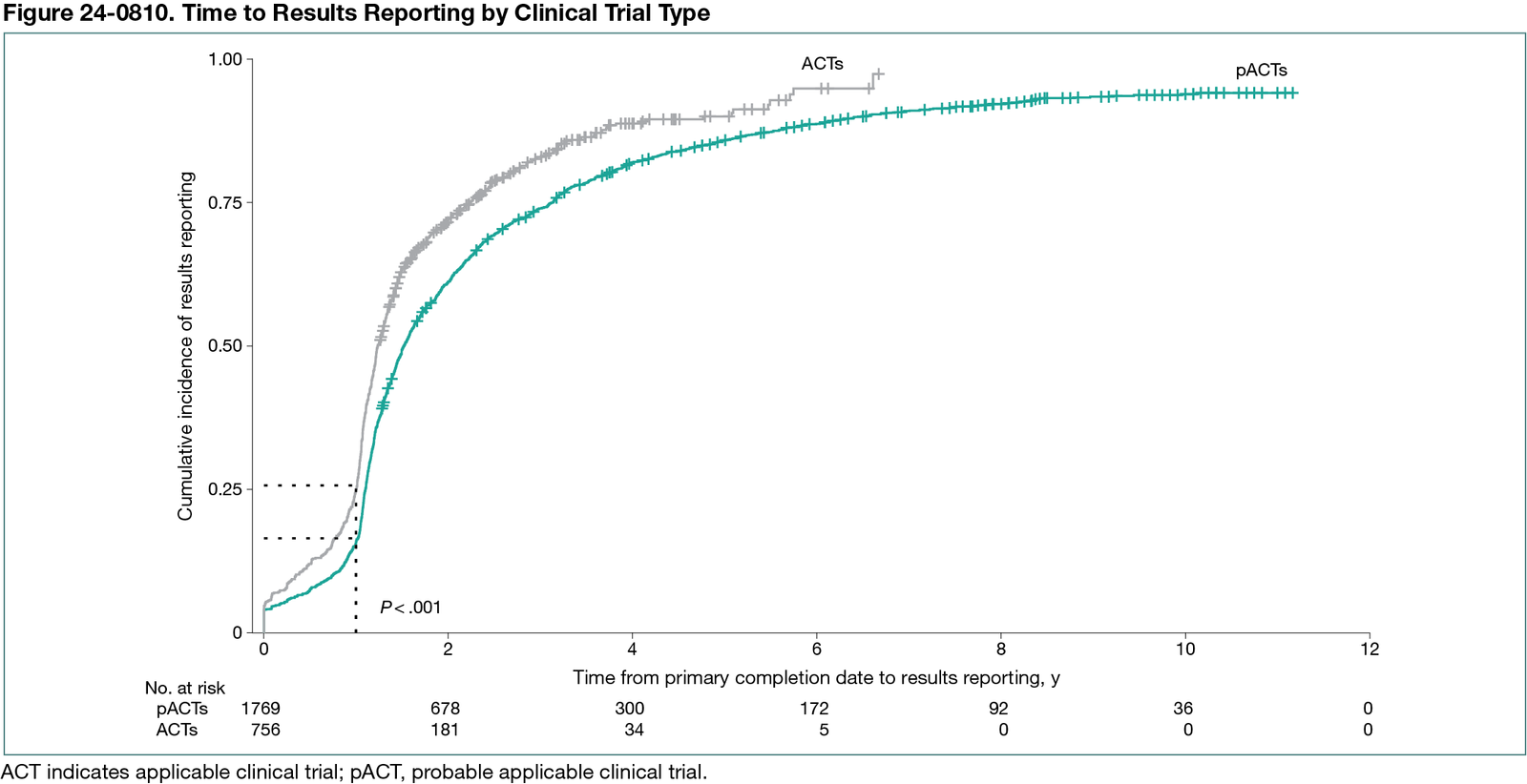
Conclusions
Studies including AMAs designated as ACTs demonstrated higher rates of reporting compliance and shorter delays in the reporting of overdue results. While this analysis provides initial insights, limitations related to timeline and sample scope suggest that broader investigations are needed to fully evaluate the policy’s impact.
1University of California, Berkeley, CA, US; 2University of Santa Cruz, Santa Cruz, CA, US; 3Harvard-MIT Center for Regulatory Science, Harvard Medical School, Boston, MA, US, maximiliansiebert91@gmail.com.
Conflict of Interest Disclosures
None reported.
Additional Information
The protocol for this study was registered at https://osf.io/zp8ug/.
