Abstract
Adherence to the ICMJE Prospective Registration Policy Among Trials Published in High-Impact Specialty Society Journals
Anand D. Gopal,1 Joshua D. Wallach,2 Jenerius A. Aminawung,1 Gregg Gonsalves,3 Rafael Dal-Ré,4 Jennifer E. Miller,5,6 Joseph S. Ross2,7
Objective
To evaluate adherence to the International Committee of Medical Journal Editors’ (ICMJE) prospective registration policy, identify the frequency of registrations that occurred late enough to potentially permit protocol modifications based on premature examination of collected data, and determine characteristics associated with timely registration among clinical trials published in high-impact journals associated with US professional medical societies.
Design
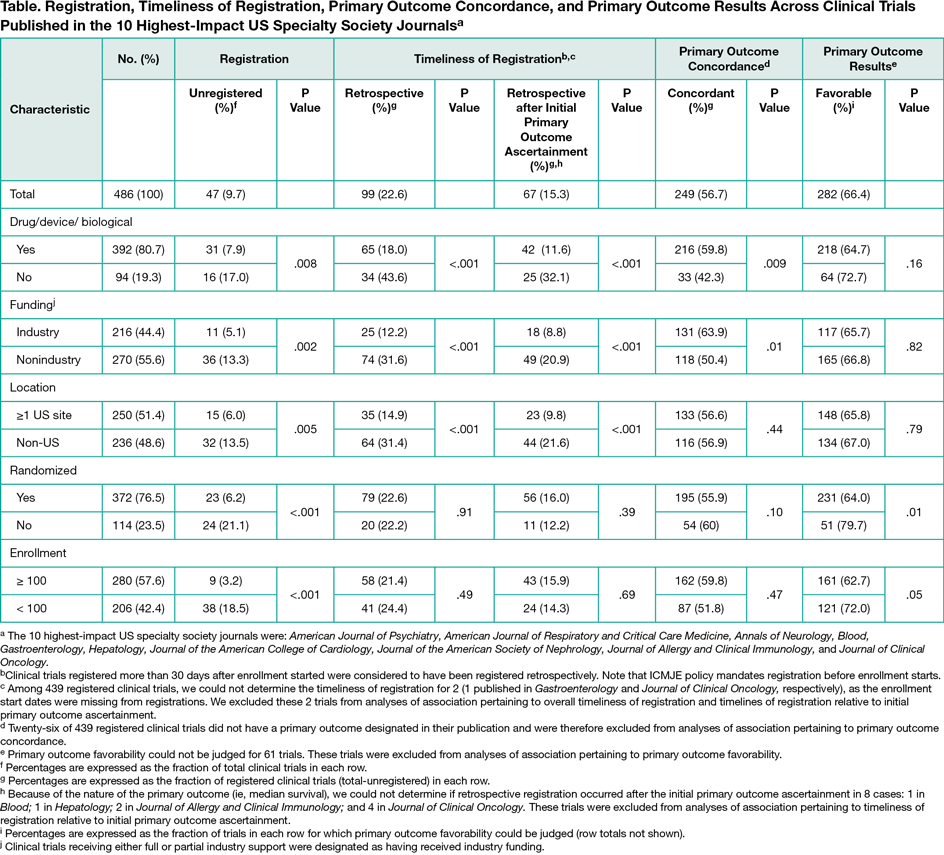
We conducted a cross-sectional analysis of the 50 most recently published clinical trials that reported primary results in the 10 highest-impact US specialty society journals between January 1, 2010 and December 31, 2015. We used descriptive statistics to characterize the proportions of clinical trials that were: registered on any of the 16 registries accepted by ICMJE; registered retrospectively; registered retrospectively potentially after initial ascertainment of primary outcomes; with concordant published and originally registered primary outcomes; and reporting favorable results, overall and stratified by journal and trial characteristics. χ2 analyses were performed with a corrected type I error of 0.006 to assess differences in registration by journal and trial characteristics.
Results
Among 6869 original research reports, we identified 472 articles reporting the primary results for 486 clinical trials. Of these 486 trials, 47 (10%) were unregistered, with proportions differing across journals. Among 439 registered clinical trials, 340 (77%) were registered prospectively and 99 (23%) retrospectively. Sixty-seven (15% of all registered trials) of these 99 retrospectively registered trials were registered late enough to have potentially permitted premature examination of primary outcome data ascertained among participants enrolled at inception. Among 413 clinical trials that registered and published at least 1 primary outcome, 109 (26%) published primary outcomes that differed from those first registered and 55 (13%) registered primary outcomes that were too poorly specified to permit comparison with published outcomes. Unregistered clinical trials were more likely to report favorable results than were registered clinical trials (89% vs. 64%; P = .004) irrespective of registration timing. FDA-regulated interventions, US-based studies, and industry funding were each associated with timely registration (Table).
Conclusions
Adherence to ICMJE prospective registration policy remains sub-standard, even among the highest impact journals associated with US professional medical societies. These journals published unregistered trials and trials registered late enough to have potentially experienced unaccounted protocol modifications after observation of primary outcomes.
1Yale University School of Medicine, New Haven, CT, anand.gopal@yale.edu; 2Center for Outcomes Research and Evaluation, Yale-New Haven Hospital and Collaboration for Research Integrity and Transparency, Yale University, New Haven, CT; 3Yale School of Public Health, New Haven, CT; 4Epidemiology Unit, Health Research Institute-Fundación Jiménez Díaz University Hospital, Universidad Autónoma de Madrid, Madrid, Spain; 5Division of Medical Ethics, Department of Population Health, New York University School of Medicine, New York, NY; 6Bioethics International; 7Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
Conflict of Interest Disclosures:
Mr Gopal receives research support through the Yale University School of Medicine Office of Student Research. Drs Wallach, Gonsalves, and Ross receive research support through Yale University from the Laura and John Arnold Foundation to support the Collaboration for Research Integrity and Transparency at Yale. Dr Miller also receives research support from the Laura and John Arnold Foundation. Dr Ross receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Centers of Medicare and Medicaid Services to develop and maintain performance measures that are used for public reporting, from Medtronic, Inc. and the US Food and Drug Administration (FDA) to develop methods for postmarket surveillance of medical devices, from the FDA as part of the Centers for Excellence in Regulatory Science and Innovation (CERSI) program, and from the Blue Cross Blue Shield Association to better understand medical technology evaluation.
Funding Support:
This project was not supported by any external grants or funds.
Acknowledgments:
The authors would like to thank Alexander R. Bazazi, and Joseph Herrold, for their efforts in drafting earlier versions of the study protocol. Mr Bazazi and Dr Herrold were not compensated for their contributions to this work.