Abstract
A Screening Checklist to Assess Data Integrity and Fabrication in Randomized Clinical Trials
Ben W. Mol,1,2 Shimona Lai,1 Ayesha Rahim,1 Wentao Li1
Objective
Because randomized clinical trials (RCTs) inform clinical guidelines and can directly influence patient care, it is important to ensure that the data behind their conclusions are trustworthy. We aimed to develop a checklist to screen RCTs for possible data fabrication at submission or prior to inclusion in meta-analyses.
Design
The development of this screening tool was adapted from the 4-stage approach proposed by Moher et al1 for reporting guidelines, including defining the scope, reviewing the evidence base, suggesting a list of items from piloting, and holding a consensus meeting as part of a Delphi method. The initial checklist was set up by a smaller core group based on the authors’ experience assessing problematic RCTs for several years. The checklist was then piloted in a Delphi panel of 20 stakeholders, including clinicians, reviewers, journal editors, and evidence synthesis specialists. Using a set of 15 articles, 8 of which were known to have fabricated data, each member was asked to score 3 articles with the checklist. Results were then discussed in 2 Delphi sessions.
Results
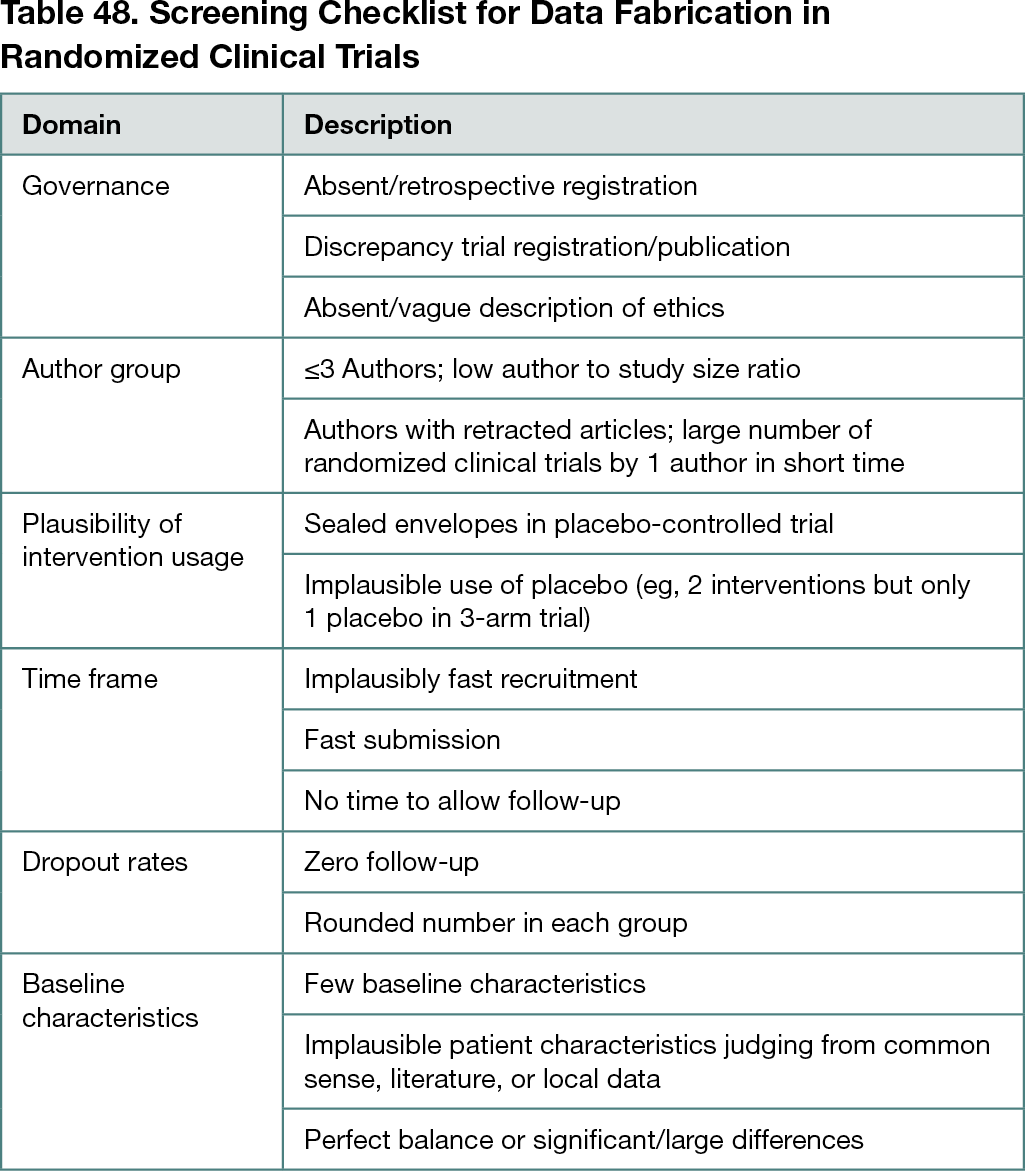
The screening checklist had 7 domains and is detailed in Table 48. The group proposed that a positive screen be defined by 2 or more items present.
Conclusions
This is the first checklist developed in a formal process to detect possible data fabrication in RCTs. If a study is assessed and found to be suspicious, reviewers can consider a more thorough investigation into the data integrity issues identified, including assessment of original data. This checklist may help editors, publishers, and researchers to screen for data fabrication in RCTs in an objective manner.
Reference
1. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLOS Medicine. 2010;7(2):e1000217. doi.org/10.1371/journal.pmed.1000217
1Department of Obstetrics and Gynaecology, Monash University, Clayton, Australia, ben.mol@monash.edu; 2Aberdeen Centre for Women’s Health Research, Institute of Applied Health Sciences, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK
Conflict of Interest Disclosures
Ben W. Mol is supported by the National Health and Medical Research Council (grant GNT1176437), reports consultancy for ObsEva and Merck, and receives travel support from Merck. No other disclosures were reported.