Abstract
Evaluation of a Software Tool and Process to Assist Authors With Reporting Ethical Requirements of Studies
Tracy Ronan,1 Alice Ellingham1
Objective
Authors are increasingly required to provide in-depth information when submitting articles, including ethics statements for Human, Animal, and Field Studies (such as the name of the institutional review board, the patient consent type, and the clinical trial registration details and dates). In our experience, authors, especially nonnative English-language speakers, can find formulating these statements a complex process. Designed as an initial proof of concept, we aimed to discover if helping authors with generating ethics statements before submitting articles could speed up the submission process and provide a clearer statement.
Design
Ethics statements were collected over a 3-day period from 13 biomedical journals. Each statement was checked against the journal’s criteria and marked as passed or failed. All failed statements were returned to the authors as per the individual journal’s processes. These were tracked to capture the additional information provided by the author to generate a statement via www.ethicsgen.com. These statements were then checked against the journal’s criteria to determine whether the author would have provided an acceptable statement at submission having used the author’s tool. All statements (excluding 47 incomplete statements) were passed through www.ethicsgen.com and then the order was randomized. The first 340 statements were offered to 7 preagreed editors in a survey for them to choose either their preferred statement or “no preference.”
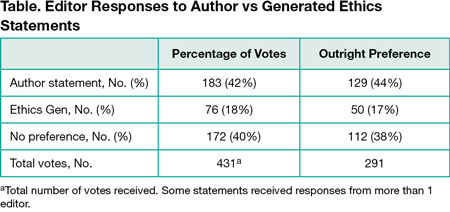
Results
Of 488 statements, 86 (17.6%) would not have passed their journal’s ethics criteria at the stage of checking and were returned to the author. Of these, 16 have since been resubmitted and the generated ethicsgen statement would have been accepted in these cases, demonstrating that the original submission would have passed the ethics check had the author had access to the tool before submitting. The other 70 statements will continue to be resubmitted by authors, but the time frame for this study was too short to include them. Four editors completed the survey, with 291 of the offered 340 statements receiving at least 1 response (Table).
Conclusion
While these results suggest that aiding authors to produce a statement may help some authors, this limited study does not provide enough detail to determine what level of time saving this would give an editorial office. A more in-depth study over a longer period would enable us to evaluate time savings or other efficiencies. Further work could identify which statements might benefit from this author tool and suggest how to provide additional support to authors.
1Editorial Office Ltd, Overton, Hampshire, UK, tracy.ronan@editorialoffice.co.uk
Conflict of Interest Disclosures:
Ms Ellingham is a codeveloper of Ethics Gen.
Funding/Support:
None reported.