Perspectives on Responsibilities in Receipt and Secondary Use of Data in Health Research
Abstract
Kylie E. Hunter,1 Aidan C. Tan,1 Angela C. Webster,1 Daniel G. Hamilton,2,3 Myra Cheng,4 Lee Jones,5 Sol Libesman,1 Salma Fahridin,1 Antonio Laguna Camacho,6 Rui Wang,7 Anna Lene Seidler1
Objective
Despite strong in-principle support for the concept of data sharing for health research, in practice it is often difficult to find, access, and reuse data.1 In the absence of agreed-on global standards, this study sought to determine the responsibilities of recipients of health research data and propose how these responsibilities can be met.
Design
A qualitative study involving an online focus group was conducted in December 2021 at the Association for Interdisciplinary Meta-research and Open Science (AIMOS) conference. All conference delegates were eligible to attend, and targeted invitations were sent to known data-sharing experts. The conference was open to all and offered free attendance. The focus group involved discussion of 3 case studies of different data-sharing scenarios in health research (an individual participant data meta-analysis, study replication, and secondary analyses) and a general discussion prompted by key questions. Participants contributed by speaking in the video call or by typing in real time on shared Google documents. Afterward, notes and recording transcripts were collated into categories using thematic analysis and shared with attendees for review and further input. Primary outcomes were the responsibilities of data recipients across the design, conduct, analyses, and reporting stages of their research. Secondary outcomes included how data providers may support data recipients to meet these responsibilities.
Results
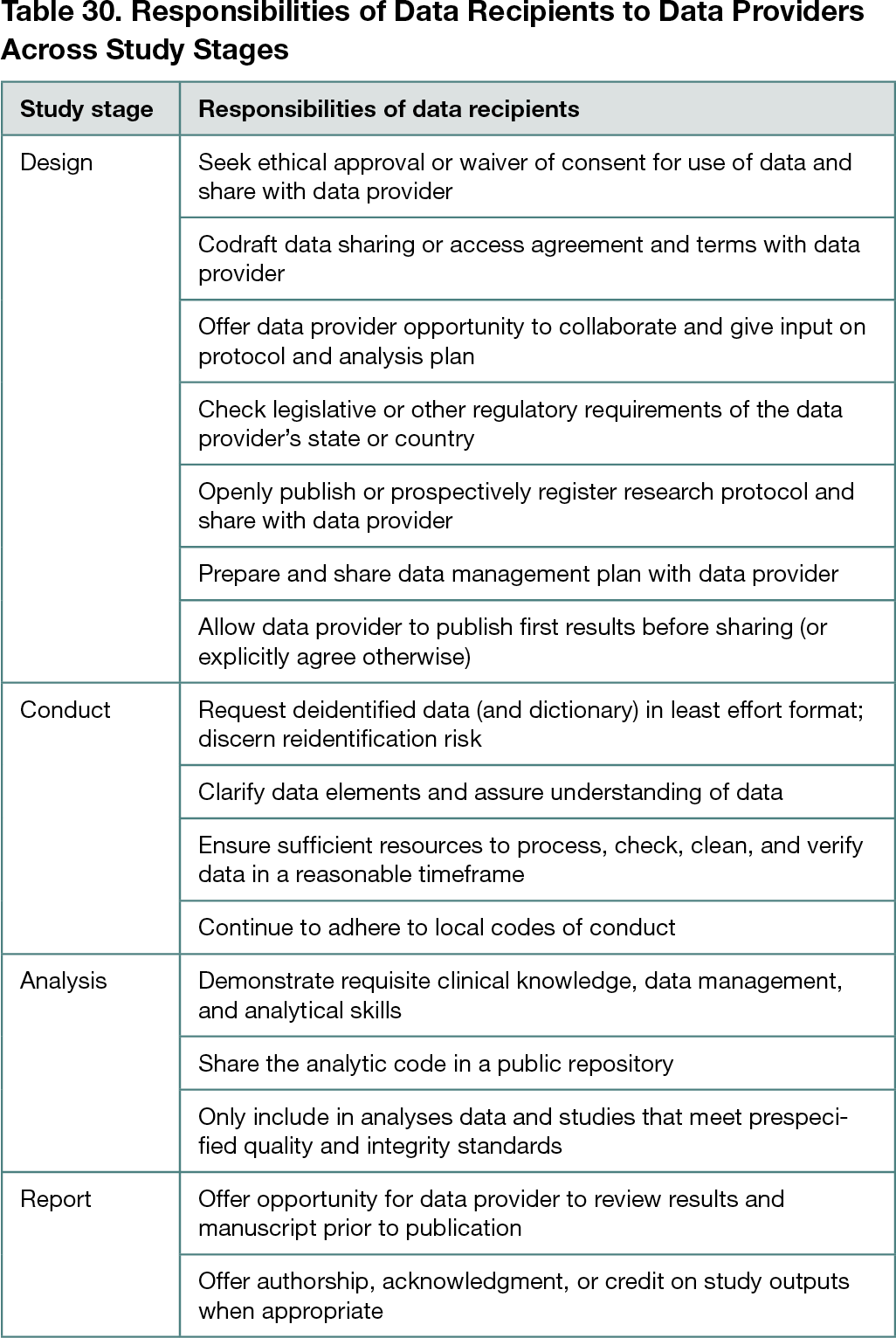
The 2-hour focus group discussion was attended by 16 conference delegates (including 3 facilitators; 11 delegates agreed to coauthor this abstract). Although AIMOS is a multidisciplinary conference, most attendees had health care–related roles across various fields, including epidemiology, statistics, evidence synthesis, policy, ethics, public health, data management, oncology, psychology, nutrition, clinical trials, and administration. Most participants cited a university as their primary employer. Analyses revealed several recurring themes across the data-sharing scenarios, which were grouped into recommendations (Table 30). Recommendations included that data recipients need to prioritize the protection of participant privacy and should proactively share a secure data management plan and evidence of ethical approval with the data provider. Additionally, data recipients should allay concerns about potential data misuse by demonstrating they have sufficient resources and expertise to process, check, and analyze data. They should also protect the interests of data providers by allowing them to publish their results before sharing data, offering streamlined data-sharing pathways, and inviting them to contribute to related outputs. Data providers could support recipients by planning for data sharing during study design, including funding support, local legislation, intellectual property, commercial-in-confidence information, and by preparing a data dictionary.
Conclusions
This study provides clarity around responsibilities of data recipients to address common concerns of data providers on data misuse and privacy. Several recommendations were derived on how data providers can support recipients to bridge the gap between high support for data sharing and low in-practice compliance.
Reference
1. Tan AC, Askie LM, Hunter KE, Barba A, Simes RJ, Seidler AL. Data sharing—trialists’ plans at registration, attitudes, barriers and facilitators: a cohort study and cross-sectional survey. Res Syn Meth. 2021; 1-17. doi:10.1002/jrsm.1500
1National Health and Medical Research Council (NHMRC) Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia, kylie.hunter@sydney.edu.au; 2MetaMelb Research Group, School of BioSciences, The University of Melbourne, Melbourne, Australia; 3Melbourne Medical School, Faculty of Medicine, Dentistry & Health Sciences, The University of Melbourne, Melbourne, Australia; 4Australian Research Data Commons , Australia; 5School of Public Health and Social Work, Queensland University of Technology, Brisbane, Australia; 6Autonomous University of the State of Mexico, Toluca, Mexico; 7Department of Obstetrics & Gynaecology, Monash Health, Monash University, Melbourne, Australia
Conflict of Interest Disclosures
Kylie E. Hunter and Antonio Laguna Camacho lead or colead several large individual participant data meta-analyses. Kylie E. Hunter and Angela C. Webster are associate convenors of the Cochrane Prospective Meta-Analysis Methods Group, and Antonio Laguna Camacho is coconvenor. Daniel G. Hamilton is a member of the Association for Interdisciplinary Meta-research and Open Science board. No other disclosures were reported.
Funding/Support
Kylie E. Hunter received research funding support via 2 scholarships administered by the University of Sydney (Postgraduate Research Supplementary Scholarship in Methods Development [SC3504] and Research Training Program Stipend [SC3227]). Anna Lene Seidler received a National Health and Medical Research Council Investigator Grant (GNT2009432).
Role of the Funder/Sponsor
The funders had no role in any of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the abstract; and decision to submit the abstract for presentation.
Acknowledgment
We warmly acknowledge the contributions of all those who attended the focus group discussion.
