Abstract
Evaluation of the ClinicalTrials.gov Results Database and Its Relationship to the Peer-Reviewed Literature
Deborah A. Zarin,1 Tony Tse,1 Rebecca J. Williams,1 Thiyagu Rajakannan,1 Kevin M. Fain1
Objective
As of February 22, 2017, ClinicalTrials.gov contained summary results for 24,377 studies and received 160 new submissions weekly. We estimate that US academic medical centers are required to report more than half of their sponsored trials to ClinicalTrials.gov under federal policies. We previously estimated that one-half of registered studies with results posted on ClinicalTrials.gov lacked results publications. It is critical to continue assessing the degree to which this database meets its intended goals. The objective of this study was to assess the potential scientific impact of the ClinicalTrials.gov results database using our 2013 evaluation framework.
Design
We analyzed 2 samples of ClinicalTrials.gov results data to assess the impact on the available evidence base.
Results
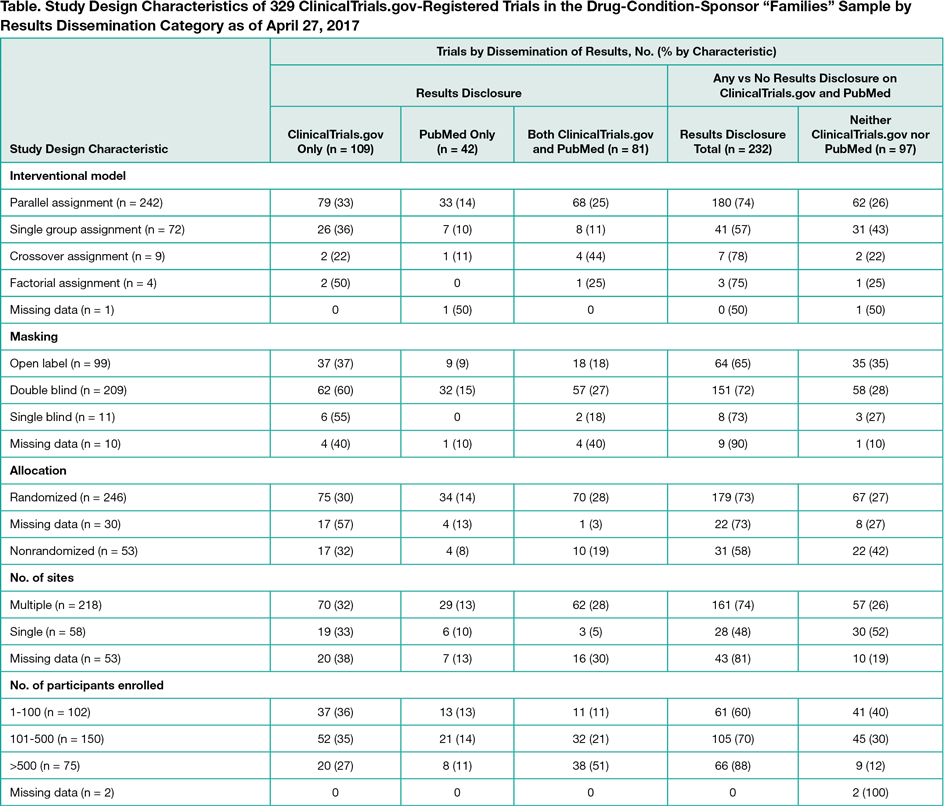
On February 10, 2017, 10,464 of 24,251 posted results (43%) had links to PubMed. Because not all publications are automatically linked and not all linked publications report results, we manually examined a random sample of 100 sets of posted results listing study completion dates in 2014. Of these, 28 had at least 1 results publication prior to results posting, 15 had a results publication after results posting, and we could not identify results publications for 57 studies. We also identified examples of how publications leveraged the information on ClinicalTrials.gov. To further examine the potential impact on selective publication, we evaluated drug-condition-sponsor “families.” We identified 329 registered, industry-funded, phase 2 through 4, US trials completed or terminated from 2007 through 2009, representing 88 drugs and 96 unique drug-condition-sponsor families (eg, Amgen-sponsored trials of alendronate for osteoporosis). Ideally, summary results for all trials in all families would be publicly available. As of December 1, 2014, of 329 trials, 109 (33%) had results posted on ClinicalTrials.gov only, 42 (13%) available from PubMed only, 81 (25%) available from both, and 97 (29%) in neither (Table). Overall, 45 of the 96 drug-condition-sponsor families had results available for all 144 trials from at least 1 source, 18 families involving a total of 48 trials had no results available, and 15 families had results disclosed on ClinicalTrials.gov only.
Conclusions
Between 33% (109 of 329) and 57% (57 of 100) of completed or terminated ClinicalTrials.gov-registered trials have posted results but no corresponding PubMed-cited results articles. These findings suggest that ClinicalTrials.gov provides a unique source of results for substantial numbers of trials.
1ClinicalTrials.gov, National Library of Medicine, National Institutes of Health, Bethesda, MD, USA, dzarin@nih.gov
Conflict of Interest Disclosures:
All authors work for ClinicalTrials.gov as full-time employees of the National Library of Medicine. Dr Zarin is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract.
Funding/Support:
This work was supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health. The National Library of Medicine has approved this submission.