Abstract
Data Sharing Statement Modifications in Manuscripts Reporting Interventional Clinical Trials Sponsored by a Global Biopharmaceutical Company
Colin McKinnon,1 Jesse Potash,2 Callan Fromm,2 Teodor G. Paunescu,3 Hajin Yang,2 Ingeborg Cil,4 Friedrich Maritsch,4 Borislava Pavlova,4 Valérie Philippon2
Objective
Following guidance on data sharing from the International Committee of Medical Journal Editors,1 Takeda requires the inclusion of a data sharing statement in most manuscripts reporting results from interventional clinical trials. The standard version of this statement indicates that deidentified patient data will be made available within 3 months to researchers who provide a methodologically sound proposal. The implementation of this policy for data sharing by request was assessed by reviewing published articles to determine whether a data sharing statement was included, modified, or not included, and the reason(s) for not sharing data were identified.
Design
To identify articles for inclusion in this study, an internal publication management system was used. Articles published online between July 2020 and November 2021 reporting primary or secondary analyses from clinical trials sponsored by Takeda and reviewed by an internal clinical trial transparency (CTT) team were included in this analysis. Each article was assigned to 1 of the following categories: inclusion of standard data sharing statement, inclusion of modified data sharing statement, statement indicating there is no plan to share data, or no data sharing statement.
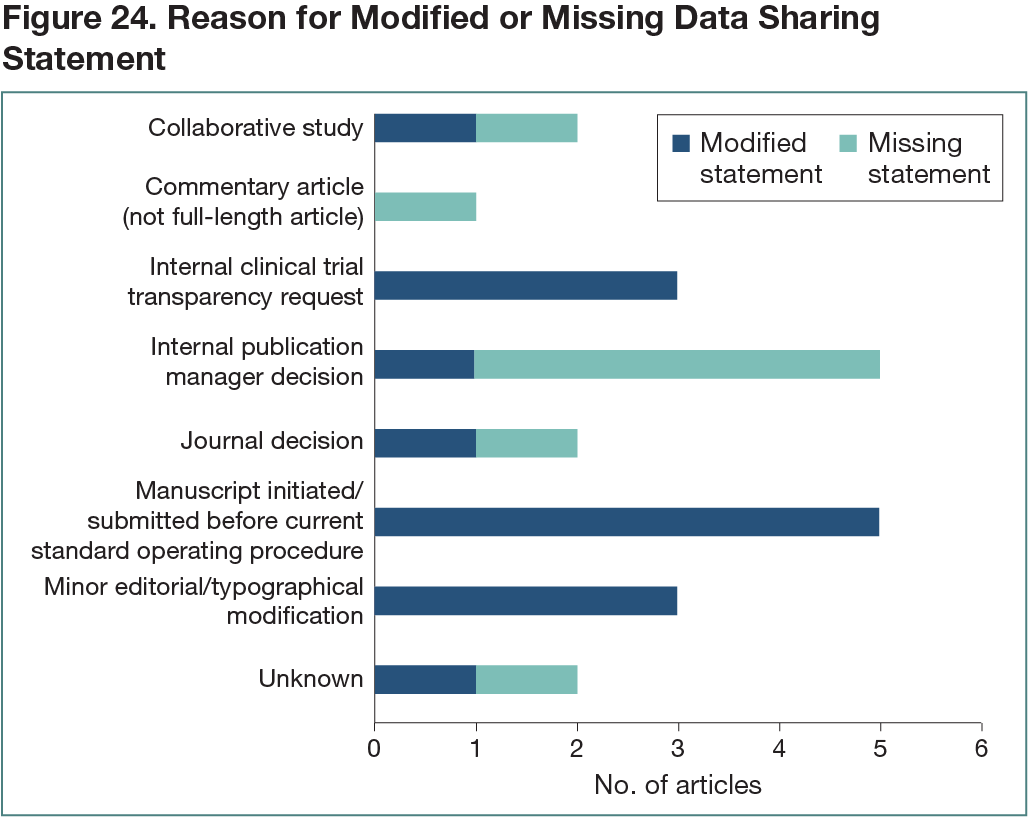
Results
A total of 36 interventional clinical trial manuscripts reviewed by the CTT team were included in the analysis. Of these, 26 (72%) included a statement outlining data availability: 11 (31%) used the recommended standard data sharing statement as outlined in the internal policy, while 15 (42%) contained a modified statement on data availability, ranging from minor editorial amendments (generally consistent with the standard statement) to substantial differences in wording (due to specific journal requirements) (Figure 24). Only 2 articles (6%) contained a statement indicating that there was no plan to share the data—both were reporting results from rare disease studies involving very small numbers of patients, and the reason was concern regarding the risk of patient reidentification due to the number of patients. No data sharing statement was included in 8 articles (22%) for various reasons (Figure 24). Of the journals that published articles without a data sharing statement, 3 had policies requiring inclusion of a data sharing statement, while the others had policies allowing and/or encouraging inclusion of a data sharing statement.
Conclusions
Sharing underlying data sets of published studies is important to promote transparency and facilitate new research. Following the adoption of a standardized approach to data sharing by request, most interventional clinical trial articles in this analysis contained a data sharing statement. In a few cases (n = 2), the statement indicated that data would not be shared to protect patient privacy in situations in which deidentification of patient-level data was not possible.
Reference
1. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Ann Intern Med. 2017;167(1):63-65. doi:10.7326/M17-1028
1Takeda Pharmaceuticals International AG, Zürich, Switzerland; 2Takeda Development Center Americas, Inc, Cambridge, MA, USA, jesse.potash@takeda.com; 3Takeda Pharmaceuticals U.S.A., Inc, Lexington, MA, USA; 4Baxalta Innovations GmbH, Vienna, Austria
Conflict of Interest Disclosures
Colin McKinnon, Jesse Potash, Teodor G. Paunescu, Borislava Pavlova, and Valérie Philippon are current or past (V.P.) employees of Takeda and are Takeda stockholders. Callan Fromm is a consultant for the Global Publications Department at Takeda. Ingeborg Cil and Friedrich Maritsch are consultants for Takeda’s Clinical Trial Transparency Department at Baxalta (a Takeda company). Hajin Yang is a postdoctoral fellow at Takeda.
Funding/Support
This study was sponsored by Takeda Development Center Americas, Inc.
Role of the Funder/Sponsor
The sponsor played a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the abstract; and decision to submit the abstract for presentation.