Replication and Impact of Positive Secondary Findings in Negative or Neutral Cardiovascular Trials
Abstract
Sina Rashedi,1 Farbod Zahedi Tajrishi,2 Ashkan Hashemi,3 Isaac Dreyfus,4 Nicholas Varunok,5 John Burton,6 Seng Chan You,7 Bjorn Redfors,8,9 Gregory Piazza,1,10 Joshua D. Wallach,11 Lesley Curtis,12 Sanjay Kaul,13 David J. Cohen,14,15 Roxana Mehran,16 Mitchell S. V. Elkind,17 Flavia Geraldes,18 Joseph S. Ross,19,20 Jane A. Leopold,10 Harlan M. Krumholz,19,21 Gregg W. Stone,16 Behnood Bikdeli1,10,19
Objective
A large number of cardiovascular randomized clinical trials (RCTs) do not meet their primary outcome (neutral or negative trials). Some of these negative/neutral RCTs show positive secondary findings. We aimed to assess the proportion of cardiovascular disease– or stroke-related RCTs with negative or neutral primary results but positive secondary findings among all negative/neutral cardiovascular RCTs published in the highest-impact medical journals and to evaluate whether these findings were pursued in subsequent confirmatory RCTs or influenced clinical practice recommendations or regulatory decisions.
Design
We searched PubMed to identify cardiovascular RCTs published in 3 major clinical journals (NEJM, The Lancet, and JAMA) between 2014 and 2019. Negative/neutral RCTs with positive secondary findings were defined as those that did not meet their (co)primary outcome(s) (P > .05) but showed positive effects in prespecified subgroup analyses for the primary outcome and/or for prespecified secondary outcomes among the entire study population. Secondary outcomes and subgroup analyses were considered if they were reported as prespecified in the trial publications. We investigated whether these positive secondary findings were examined in subsequent confirmatory RCTs via PubMed searches or directly resulted in changes in either professional society recommendations or regulatory authority endorsements in the US or Europe through December 2024. Two investigators independently performed the literature search and data extraction, with discrepancies resolved by a third investigator.
Results
Overall, 435 cardiovascular RCTs were identified; the median time from the index trial to December 2024 was 7.5 (IQR, 5.5-9.5) years. Of these 435 RCTs, the primary outcome(s) were not met in 174 trials (40.0%), of which 93 (53.4%) had at least 1 positive secondary finding. Among these trials, 51 (54.8%) had only positive secondary outcomes, 20 (21.5%) had only positive findings in subgroup analyses, and 22 (23.7%) had positive findings in both secondary outcomes and subgroup analyses. Among the 93 negative/neutral RCTs with positive secondary findings, 14 (15.1%) were consequential: subsequent RCTs were performed due to positive secondary findings for 2 trials; the secondary results from 12 RCTs contributed to changes in professional society recommendations; and 2 trials contributed to changes in labeled indications (Figure 25-0928).
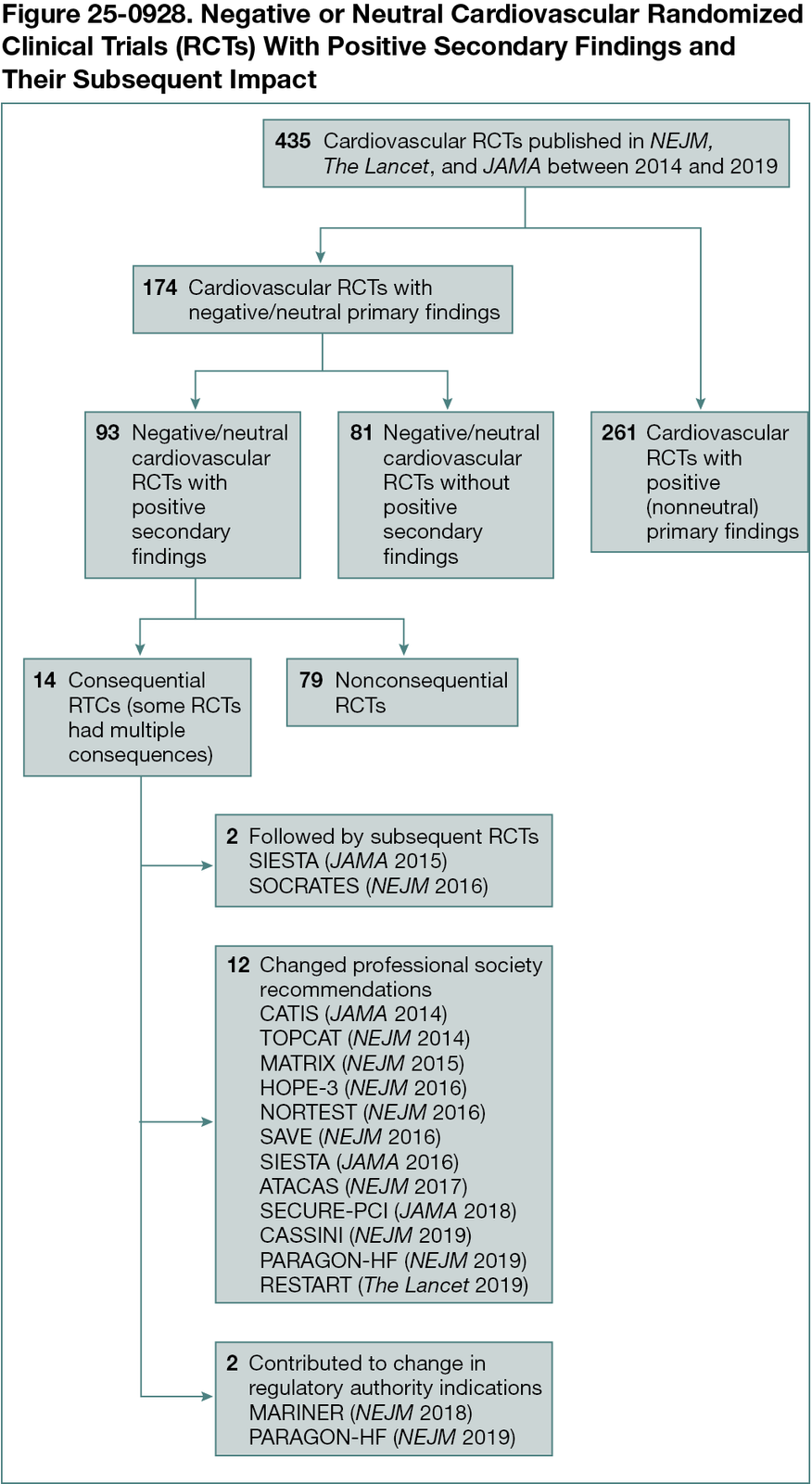
Conclusions
Although nearly half of the negative/neutral cardiovascular trials published in the highest-impact journals report positive secondary findings, only a small proportion directly influence practice or policy, and very few are followed by subsequent confirmatory RCTs. Our findings caution against overemphasizing positive secondary results from cardiovascular RCTs and underscore the need for subsequent confirmatory RCTs to evaluate positive secondary findings from otherwise negative/neutral trials. Further research is planned to assess the generalizability of these findings in major specialty cardiovascular journals and to identify trial- and outcome-level characteristics that influence the selection of positive secondary findings for replication in subsequent trials.
1Thrombosis Research Group, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, US, srashedi@bwh.harvard.edu; 2Tulane University School of Medicine, New Orleans, LA, US; 3Program for the Care and Study of the Aging Heart, Department of Medicine, Weill Cornell Medicine, New York, NY, US; 4Division of Cardiology, Department of Medicine, University of California Los Angeles, Los Angeles, CA, US; 5Vanderbilt University Medical Center, Nashville, TN, US; 6University of Southern California, Los Angeles, CA, US; 7Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea; 8Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Sweden; 9Cardiovascular Research Foundation, New York, NY, US; 10Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, US; 11Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, US; 12Duke University School of Medicine, Department of Population Health Sciences, Durham, NC, US; 13Department of Cardiology, Cedars-Sinai Medical Center, Los Angeles, CA, US; 14Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, US; 15St Francis Hospital and Heart Center, Roslyn, NY, US; 16Icahn School of Medicine at Mount Sinai, New York, NY, US; 17Department of Neurology, Columbia University Irving Medical Center, New York, NY, US; 18The Lancet, London, United Kingdom; 19YNHH/Yale Center for Outcomes Research and Evaluation (CORE), New Haven, CT, US; 20Department of General Internal Medicine, Yale School of Medicine, New Haven, CT, US; 21Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT, US.
Conflict of Interest Disclosures
Outside the submitted work, Behnood Bikdeli reported receiving the following support: a Career Development Award from the American Heart Association and VIVA Physicians (#938814), the Scott Schoen and Nancy Adams IGNITE Award, the Mary Ann Tynan Research Scientist Award from the Mary Horrigan Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital, and the Heart and Vascular Center Junior Faculty Award from Brigham and Women’s Hospital; serving as consulting expert on behalf of the plaintiff for litigation related to 2 specific brand models of IVC filters (although he has not been involved in the litigation in 2022-2025, nor has he received any compensation in 2022-2025); serving as a member of the Medical Advisory Board for the North American Thrombosis Forum (now VascuLearn Network) and on the Data Safety and Monitoring Board of the NAIL-IT trial funded by the National Heart, Lung, and Blood Institute and Translational Sciences; serving as a collaborating consultant with the International Consulting Associates and the US Food and Drug Administration in a study to generate knowledge about the utilization, predictors, retrieval, and safety of IVC filters; receiving compensation as an associate editor for NEJM Journal Watch Cardiology, as an associate editor for Thrombosis Research, and as an executive associate editor for JACC; and serving as a section editor for Thrombosis and Haemostasis without compensation.
Additional Information
Behnood Bikdeli is a co–corresponding author (behnood.bikdeli@yale.edu).
