Prevalence of Prospective Registration and Primary Outcome Discrepancies in Recently Published Randomized Controlled Trials
Abstract
Ioana Alina Cristea,1,2 Florian Naudet,2,3,4 Guillaume Cabanac,4,5 John P. A. Ioannidis2,6,7,8
Objective
An older, field-wide analysis1 of studies published between 2005 and 2017 estimated the prevalence of prospectively registered randomized controlled trials (RCTs) at 21%. Recent estimates indicated higher rates, eg, approximately 59% for rheumatology RCTs published between 2009 and 2022 in 5 International Committee of Medical Journal Editors (ICMJE) journals.2 There are no recent field-wide estimates of the prevalence of prospective registration across clinical specialties and journals and of the related outcome reporting bias (ie, discrepancies between registered and published outcomes). We report these across a randomly selected sample of recently published RCTs.
Design
We conducted a retrospective cohort study, reported following the STROBE guidelines. We searched PubMed with the Cochrane Highly Sensitive Search Strategy3 on January 15, 2025. RCTs evaluating health-related interventions and outcomes (per the ICMJE definition) published in 2024 were included. Search results were randomized using Entrez Programming Utilities and the Linux shuf command, and a sample of 1720 records was selected. One researcher (I.A.C.) manually checked titles and abstracts to select RCTs and extracted trial registration information from publications reporting on primary outcomes (POs). Prospective registration was defined as submission date to registry preceding recruitment or study start or postdating by less than 30 days. We favored actual vs estimated study start dates when available in publications or registries. For prospectively registered trials, 1 researcher (I.A.C.) extracted all POs registered and declared in publications, and tabulated all discrepancies regarding outcome domain, measurement, or time point.
Results
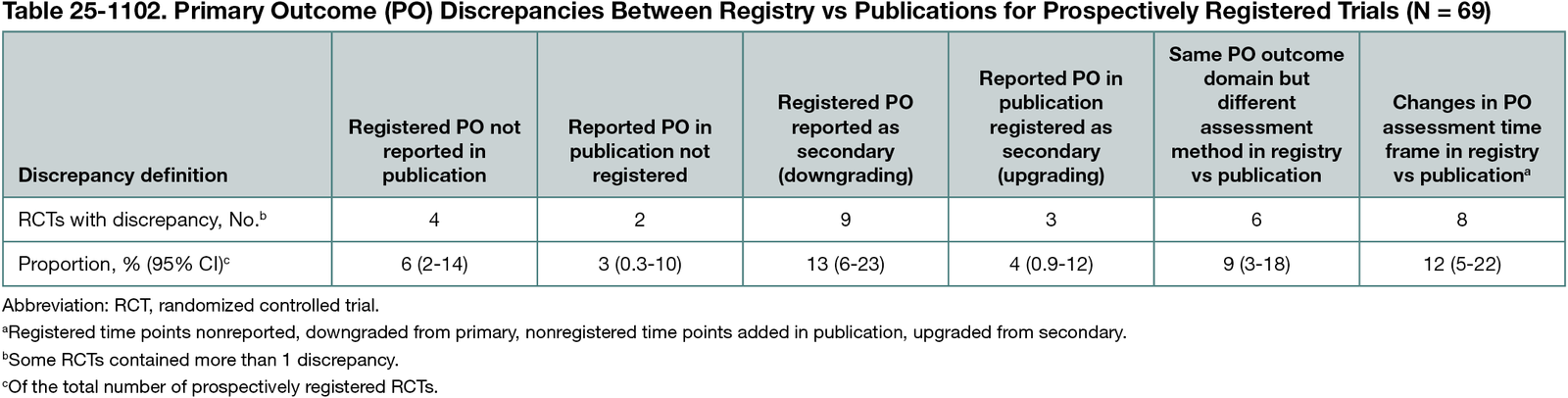
Of 324,177 records identified, 1720 were screened, leading to 136 eligible RCTs. Six trials were excluded (inaccessible full text [n = 3]; Chinese language [n = 3]). Eighty-two of 130 publications (63%) reported study start dates, with a median (IQR) of 2020 (2) (range, 2013-2023). Sixty-nine of 130 RCTs (53% [95% CI, 44%-62%]) were prospectively registered (67 in ICMJE-compliant registries), including 4 registered while recruiting. Sixty-one of 130 RCTs (47% [95% CI, 38%-56%]) were declared nonregistered (n = 4), were registered retrospectively (n = 27), indicated a registration number for another trial (n = 2), or did not mention registration (n = 28). Registration delays were a median (IQR) of 632 (533) (range, 49-1524) days. For 24 of 60 nonprospectively registered trials, journal instructions to authors explicitly required prospective registration or compliance with ICMJE or Declaration of Helsinki registration requirements. Of 69 prospectively registered trials, 6 did not declare POs in publications and 2 insufficiently specified POs in the registries, while 37 (54% [95% CI, 41%-66%]) had no substantive discrepancies between registry and publication. The remaining 24 of 69 RCTs (35% [95% CI, 24%-47%]) included 1 (n = 17), 2 (n = 6), or 3 (n = 1) types of discrepancies (Table 25-1102).
Conclusions
In a random sample of RCTs published in 2024 across clinical specialties, approximately half were prospectively registered and approximately one-third of these contained substantial changes between registered and reported primary outcomes.
References
1. Trinquart L, Dunn AG, Bourgeois FT. Registration of published randomized trials: a systematic review and meta-analysis. BMC Med. 2018;16(1):173. doi:10.1186/s12916-018-1168-6
2. Mongin D, Buitrago-Garcia D, Capderou S, et al. Prospective registration of trials: where we are, why, and how we could get better. J Clin Epidemiol. 2024;176. doi:10.1016/j.jclinepi.2024.111586
3. Lefebvre C, Glanville J, Briscoe S, et al. Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.5. Cochrane; 2024
1Department of General Psychology, University of Padova, Padova, Italy, ioanaalina.cristea@unipd.it; 2Meta-Research Innovation Center at Stanford (METRICS), Stanford University, Stanford, CA, US; 3Université Rennes, CHU Rennes, Inserm, Centre d’investigation clinique de Rennes (CIC1414), service de pharmacologie clinique, Institut de recherche en santé, environnement et travail (Irset), UMR S 1085, EHESP, 35000, Rennes, France; 4Institut Universitaire de France (IUF), Paris, France; 5Université de Toulouse, IRIT (UMR 5505 CNRS), Toulouse, France; 6Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine, Stanford, CA, US; 7Department of Epidemiology & Population Health, Stanford University School of Medicine, Stanford, CA, US; 8Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA, US.
Conflict of Interest Disclosures
John P. A. Ioannidis is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract.
Funding/Support
Ioana Alina Cristea is supported by a European Research Council (ERC) Starting Grant (grant agreement: 101042701; https://cordis.europa.eu/project/id/101042701), funded by the European Union (EU). Florian Naudet received funding from the French National Research Agency, the French Ministry of Health, and the French Ministry of Research and is a work package leader in the OSIRIS project (Open Science to Increase Reproducibility in Science) and for the doctoral network MSCA-DN SHARE-CTD (HORIZON-MSCA-2022-DN-01 101120360), funded by the EU. Guillaume Cabanac received funding from the Institut Universitaire de France. John P. A. Ioannidis is supported by an unrestricted gift from Sue and Bob O’Donnell to Stanford University.
Role of the Funder/Sponsor
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the abstract.
Additional Information
John P. A. Ioannidis is a co–corresponding author (jioannid@stanford.edu).
