Open Access and Copyright License Status of Pharmaceutical Company–Supported Articles
Abstract
Elin Bevan,1 Tim Koder,1 Valérie Philippon,2 Slávka Baróniková,3 Larisa Miller,4 William Gattrell,5 Tomas Rees1
Objective
Informatics approaches have previously been used to assess open access (OA) rates for pharmaceutical company–supported publications.1 An updated method was developed using article type metadata supplied by Embase, aiming to improve differentiation between journal articles and published congress materials. Copyright license types of OA pharmaceutical company–supported journal articles were also identified.
Design
Articles from 24 pharmaceutical companies were analyzed, including the top 20 companies by revenue (as of September 2021)2 and the 4 OpenPharma participant companies outside the top 20.3 A scholarly search aggregator (Lens.org) was used to identify articles with authors affiliated with each company, a field of study of medicine, and publication in 2019 or 2020. Only publications tagged as article by Embase were included. The OA status and copyright license type were evaluated through Unpaywall (a database of OA articles). The 2019 analysis was performed using the original method based on PlumX-supplied data to establish any differences between the 2 methods. Data collection occurred from July to September 2021.
Results
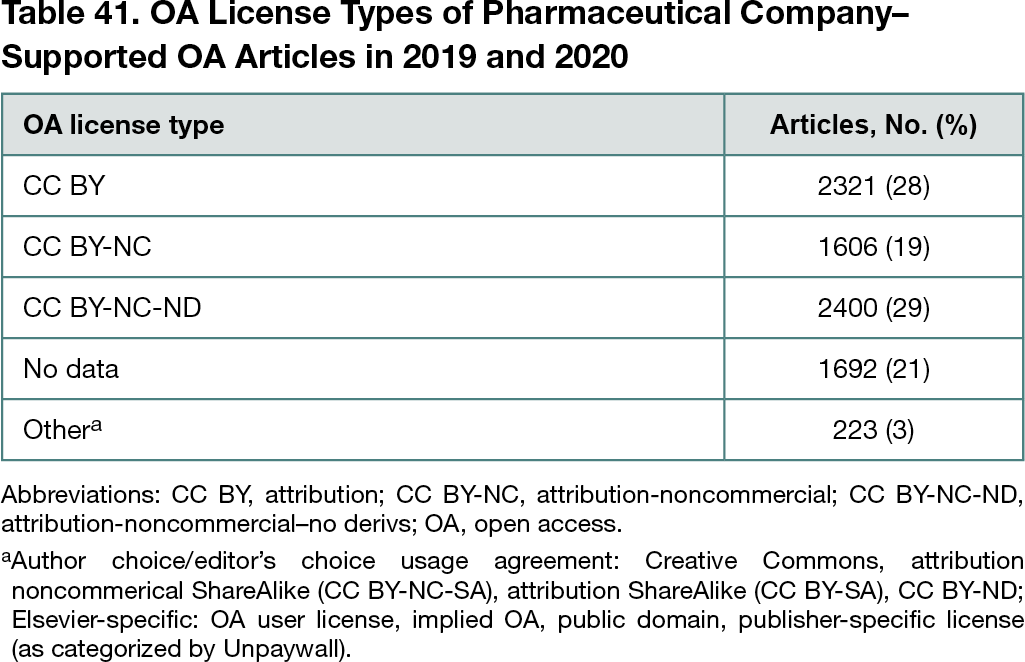
For 2019, the updated method returned fewer total articles (5093) than the original method (6900), with an overall OA rate of 76%, an increase from 69%. Analysis of 1 company with a known 100% OA rate revealed that in 2019, using the previous methods, 15 of 50 articles were classified incorrectly as non-OA; with the new method for the same data set, the number of articles was 2 of 37. The 2020 OA rate using the revised method was 77%, from a total of 5678 articles. Excluding companies with fewer than 10 articles (2019, 2 of 24; 2020, 1 of 24), the range of company OA rates was 67% to 95% in 2019 and 56% to 90% in 2020. OpenPharma company participants had higher OA rates than nonparticipants, and their OA rates increased from 2019 to 2020 by 0.5 percentage point (77.1% to 77.6%) compared with 1.5 percentage points for nonparticipants (74.6% to 76.1%). The most common copyright license types in the 8242 OA journal articles published in 2019 and 2020 combined were CC BY-NC-ND (29% of total articles) and CC BY (28% of articles) (Table 41). Unpaywall was unable to distinguish the copyright license type for 21% of OA articles.
Conclusions
The revised method improved accuracy of estimating OA rates for pharmaceutical company–supported articles by excluding incorrectly tagged publications identified with the original method, likely because Embase includes a manual step in their tagging. Almost half of the articles analyzed had a copyright license preventing commercial use. This analysis was restricted to only those articles with pharmaceutical company authors and articles tagged as “medicine”; therefore, this analysis does not encompass the full range of publications associated with the pharmaceutical industry. However, analyses using this method could help pharmaceutical companies, collaborating academic institutions, and publishers working toward greater openness and expanded reach of peer-reviewed publications.
References
1. Freeman H, Macdonald S, Baróniková S, et al. Original abstracts from the 2021 European Meeting of ISMPP. Curr Med Res and Opin. 2021;37:21-39. doi:10.1080/03007995.2021.1894669
2. Fiercepharma.com. The top 20 pharma companies by 2020 revenue. Accessed January 28, 2022. https://www.fiercepharma.com/special-report/top-20-pharma-companies-by-2020-revenue
3. OpenPharma. About us. Accessed January 28, 2022. https://openpharma.blog/about-us/
1Oxford PharmaGenesis, Oxford, UK, elin.bevan@pharmagenesis.com; 2Takeda Development Center Americas, Inc, Cambridge, MA, USA; 3Galápagos NV, Mechelen, Belgium; 4Alexion, AstraZeneca Rare Disease, Boston, MA, USA; 5Ipsen, Abingdon, UK
Conflict of Interest Disclosures
Elin Bevan, Tim Koder, and Tomas Rees are employees of Oxford PharmaGenesis. Slávka Baróniková is an employee of and may hold stock in Galápagos NV. Larisa Miller is an employee and stockholder of Alexion, AstraZeneca Rare Disease. At the time of abstract development, William Gattrell was an employee and shareholder of or held stock or stock options in Ipsen and is now an employee of Bristol Myers Squibb; Valérie Philippon was an employee and shareholder of or held stock or stock options in Takeda Development Center Americas, Inc, and is now an employee of UCB.
Funding/Support
This work was produced by representatives of OpenPharma. OpenPharma is a multisponsor collaboration facilitated by Oxford PharmaGenesis Ltd and receives sponsorship funding from Alexion Pharmaceuticals Inc, AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim International GmbH, E. R. Squibb & Sons LLC, F. Hoffmann-La Roche AG, Galápagos NV, Gilead Sciences Inc, GlaxoSmithKline Biologicals SA, Ipsen Biopharm Ltd, Janssen Global Services LLC, John Wiley & Sons Ltd, Novartis Pharma AG, Novo Nordisk A/S, Pfizer Inc, Takeda Development Center Americas Inc, and UCB Biopharma SRL.
Role of the Funder/Sponsor
The funding organizations involved in this work facilitated the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the abstract; and were involved in the decision to submit the abstract for presentation.
Acknowledgment
We thank the members and supporters of OpenPharma for their input and insightful discussions surrounding this analysis.
