Media Attention, Twitter Engagement, and Citations of COVID-19 Clinical Trial Preprints and Their Corresponding Peer-Reviewed Publications
Abstract
Emily Inwards,1 Jennifer Klavens,1 Amanda C. Adams,2 Brian W. Roberts,1 Timothy F. Platts-Mills,3 Christopher W. Jones1
Objective
To compare media attention, Twitter engagement, and citations among COVID-19–related clinical trial preprints and corresponding peer-reviewed publications.
Design
Preprints of clinical trials were included in this cross-sectional study if they reported results related to the treatment or prevention of COVID-19 and were uploaded to an open access preprint server indexed by the National Institutes of Health iSearch COVID-19 portfolio in 2020. Two investigators, including a medical librarian, independently searched Medline, Google Scholar, and Embase to identify peer-reviewed publications corresponding to the included preprints. Altmetric data were used to quantify media mentions and Twitter interactions within 3 months after publication for both preprints and peer-reviewed manuscripts. Citation counts were also recorded using Web of Science. Descriptive statistics were reported for the included trials, and linear regression was used to assess associations between study characteristics and media mentions, Twitter interactions, and citation counts.
Results
Of 22,615 preprints screened for eligibility, 145 were included. MedRxiv was the source for most eligible preprints (n =100; 69%). Peer reviewed publications matching 118 of 145 preprints (81%) were found. Sixty-eight preprints (47%) received media citations within 3 months of publication (median [IQR] number of mentions per preprint, 0 [0-9]; maximum mentions, 568), and 118 (81%) had Twitter interactions (median [IQR] tweets per preprint, 28 [4-202]; maximum mentions, 18,177). One hundred preprints (69%) were cited in published literature (median [IQR] citations, 2 [0-12]). Among 118 preprints with matching peer-reviewed publications, Altmetric and citation data were unavailable for 5 (4%). Preprints received more media mentions than the corresponding peer-reviewed publications in 33 cases (28%), equal mentions in 38 cases (32%), and fewer media mentions in 42 cases (36%). Twitter mentions were greater for preprints than peer-reviewed publications in 54 cases (46%), equal in 2 (2%), and fewer for 62 (53%). In 26 cases (22%), preprints received more citations than peer-reviewed publications; citation counts were equal in 6 cases (5%) and in 85 cases (72%), the peer-reviewed version received more citations. Study size, government funding, failure to prospectively register, and positive study results were most often associated with increased media mentions, tweets, and citations (Table 20).
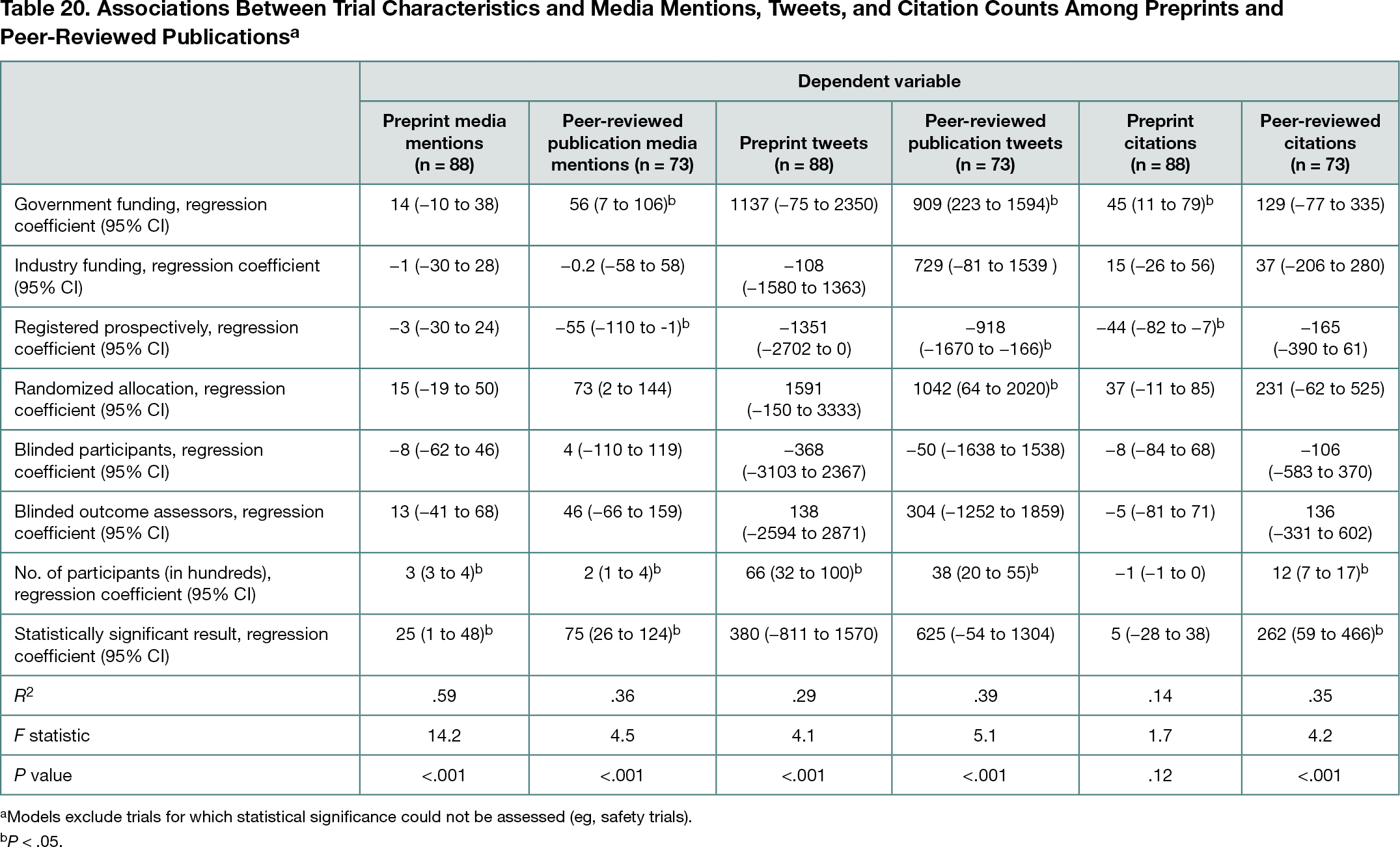
Conclusions
Although peer-reviewed publications had more media, Twitter, and citation activity than corresponding preprints in most cases, it was not uncommon for preprints to receive more attention than peer-reviewed publications. Measures of trial reliability or quality were generally not associated with increases in these metrics.
1Department of Emergency Medicine, Cooper Medical School of Rowan University, Camden, NJ, USA, jones-christopher@cooperhealth.edu; 2Medical Library, Cooper Medical School of Rowan University, Camden, NJ, USA; 3Ophirex Inc, Corte Madera, CA, USA
Conflict of Interest Disclosures
Christopher W. Jones reported receiving grants from AstraZeneca, Abbott, Vapotherm, and Ophirex outside the submitted work. Timothy F. Platts-Mills is an employee of Ophirex. No other disclosures were reported.
Funding/Support
This work was supported by the US Department of Health and Human Services (HHS) Office of Research Integrity (grant ORIIR200066-01-00).
Role of the Funder/Sponsor
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the abstract for presentation.
Additional Information
The contents of this manuscript are those of the authors and do not represent the official views of, nor an endorsement by, the Office of the Assistant Secretary for Health, the HHS, or the US government.
