Impact of ICMJE Trial Registration Policy
at 20 Years
Abstract
Julianne T. Nelson,1 Tony Tse,1 Swapna Mohan,1 Yvonne Puplampu-Dove1
Objective
The 2004 International Committee of Medical Journal Editors (ICMJE) policy required prospective registration of clinical trials as a condition for publication. Prior work reported widespread inconsistencies at earlier time points, including incomplete registration,1 retrospective or unregistered trials,2 and discrepancies between registered and published primary outcome measures.3 This analysis aims to build on previous work, now 20 years post-ICMJE policy, and expands to include the global landscape of trial publication and registration.
Design
This cross-sectional analysis assessed a convenience sample of primary trial results publications first indexed in PubMed from July to September 2024 that was randomly selected by funding category to reflect a distribution of 40% industry and 60% nonindustry.2 Each article was manually reviewed by 1 author. The journal title, first publication date, and any unique identifier from an ICMJE-recognized registry listed in the abstract or body text were extracted. Publications without trial registry information had their status confirmed via advanced search within the WHO International Clinical Trials Registry Platform (ICTRP). From a random subset of 100 sampled publications listing registry identifiers, again selected randomly for a 40% to 60% funding distribution, 1 author extracted first registration and study start dates from corresponding registration records to determine timing of registration (prospective or retrospective). The author also compared primary outcome measures (POMs) listed in publications and records. The first outcome measure mentioned was used for publications without explicitly identified POMs. Discrepancy codes were adapted from prior work.3 Registered POMs with vague terms (eg, safety) were coded as “unclear.” All data were reviewed independently by a second author and differences resolved by discussion.
Results
Of 434 overall sampled articles from 322 journal titles on October 17, 2024, 82.7% (359 of 434) disclosed registration identifiers, of which 212 (59.1%) were listed in abstracts. Of 17 represented registries, ClinicalTrials.gov (69.6% [250 of 359]), ChiCTR (7.0% [25 of 359]), and ANZCTR (3.6% [13 of 359]) were cited most frequently. Among the subset of 100 trials, 23% were retrospectively registered (Table 25-0939). Among the extracted 135 POMs, 2.2% (3 of 135) were coded as unclear and 18.5% (25 of 135) as discordant: 52.0% (13 of 25) due to published POMs missing from registration records and 28.0% (7 of 25) due to published POMs registered as secondary outcome measures. No substantive differences between trials funded by industry and nonindustry sources were observed.
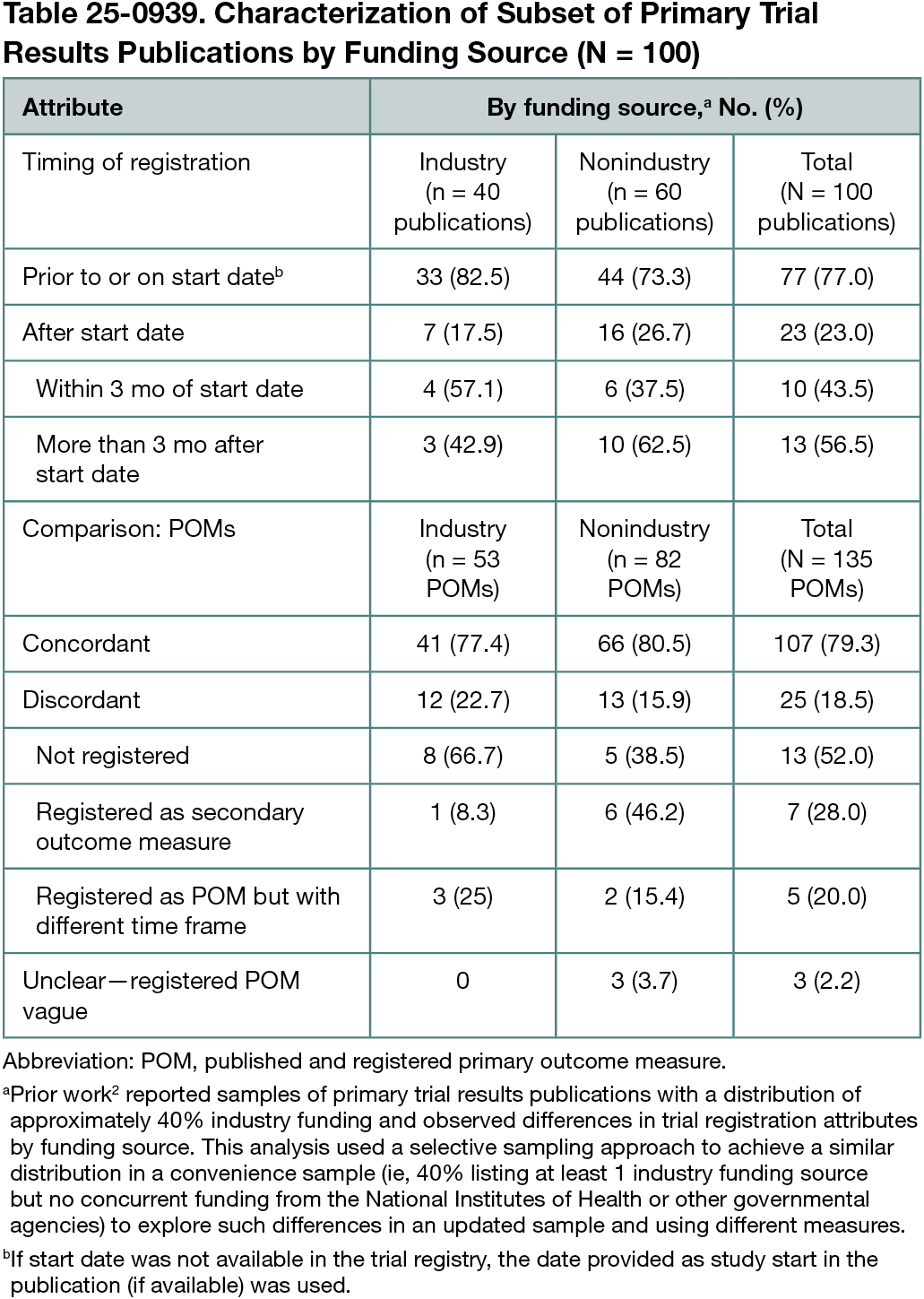
Conclusions
Twenty years after the ICMJE policy, nearly one-fifth of sampled publications did not disclose registration identifiers. Of those that did, 40% of identifiers were not displayed in abstracts (inaccessible in PubMed). Among the subset, almost one-quarter were registered retrospectively. Of published POMs, approximately one-fifth were discordant with registered information. These findings do not differ widely from previous work of 10 years ago and suggest that the policy goals of full transparency and accountability through trial registration have yet to be reached.
References
1. Viergever RF, Karam G, Reis A, Ghersi D. The quality of registration of clinical trials: still a problem. PLoS One. 2014;9(1):e84727. doi:10.1371/journal.pone.0084727
2. Gopal AD, Wallach JD, Aminawung JA, et al. Adherence to the International Committee of Medical Journal Editors’ (ICMJE) prospective registration policy and implications for outcome integrity: a cross-sectional analysis of trials published in high-impact specialty society journals. Trials. 2018;19(1):448. doi:10.1186/s13063-018-2825-y
3. Turner EH, Mulder RT, Rucklidge JJ. Is mandatory prospective trial registration working? An update on the adherence to the International Committee of Medical Journal Editors guidelines across five psychiatry journals: 2015-2020. Acta Psychiatr Scand. 2021;144(5):510-517. doi:10.1111/acps.13353
1The National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, MD, US, julianne.nelson@nih.gov.
Conflict of Interest Disclosures
None reported.
Funding/Support
This work was supported by the National Center for Biotechnology Information of the National Library of Medicine, National Institutes of Health.
Role of the Funder/Sponsor
The funder supported the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the abstract; and decision to submit the abstract for presentation.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the National Institutes of Health.
Additional Information
Swapna Mohan and Yvonne Puplampu-Dove reported that they conducted this work under contract with ICF International Inc.
