Abstract
Disclosure of Results of Clinical Trials Sponsored By Pharmaceutical Companies
Slavka Baronikova,1 Jim Purvis,2 Christopher Winchester,2,3 Eric Southam,2 Julie Beeso,2 Antonia Panayi1
Objective
To evaluate disclosure of clinical trials sponsored by pharmaceutical companies.
Design
We used TrialsTracker to identify interventional phase 2-4 clinical trials that were registered on ClinicalTrials.gov; completed between 2006 and 2015; sponsored by the top 50 pharmaceutical companies (defined by 2014 global sales using EvaluatePharma); and that had results disclosed by April 2017, where disclosure is defined as registered on ClinicalTrials.gov or published in articles indexed on PubMed. We report the proportion of trials with disclosed results overall; by company membership in the European Federation of Pharmaceutical Industries and Associations (EFPIA) and Pharmaceutical Research and Manufacturers of America (PhRMA) and by industry vs nonindustry sponsorship.
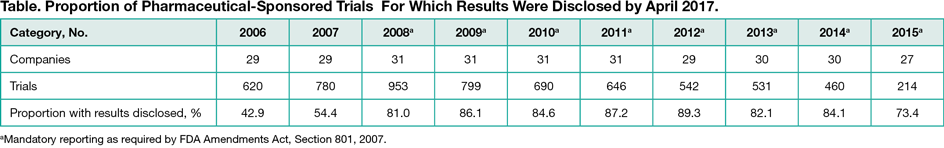
Results
Among the top 50 companies, 31 (62.0%) met inclusion criteria and were represented in TrialsTracker: 25 (80.6%) were EFPIA/PhRMA members and 6 (19.4%) nonmembers (generally medical device, generic drug, and non-EU/US companies). Among 6235 trials registered and completed by these companies between 2006 and 2015, results were disclosed for 4761 (76.4%), with the proportion rising from 42.9% in 2006 to approximately 80.0% from 2008 onwards (Table). The proportion of trials with results disclosed was similar for those sponsored by PhRMA/EFPIA members (1361 of 5697 [76.1%]) and nonmembers (113 of 538 [79.0%]). Of all clinical trials identified in TrialsTracker, results were disclosed for 74.0% of all pharmaceutical-industry sponsors and 45.7% of non-industry sponsors.
Conclusions
The pharmaceutical industry has disclosed the results of three-quarters of trials completed between 2006 and 2015. Because TrialsTracker excludes sources other than ClinicalTrials.gov (eg, company websites), this figure may be an underestimate.
1Shire, Switzerland GmbH, Zug, Switzerland, sbaronikova-c@shire.com; 2Oxford PharmaGenesis Ltd, Oxford, UK; 3Department of Medicine, Pharmacy and Health, Durham University, Durham, UK
Conflict of Interest Disclosures:
S Baronikova is a consultant to Shire Switzerland GmbH. A Panayi is an employee of Shire Switzerland GmbH. J Beeso, J Purvis, E Southam, and C Winchester are employees of Oxford PharmaGenesis Ltd, Oxford, UK. C Winchester owns shares in Oxford PharmaGenesis Holdings Ltd and AstraZeneca Ltd, and is a Director of Oxford PharmaGenesis Ltd and Oxford PharmaGenesis Holdings Ltd.
Funding/Support:
Funding was provided by Oxford PharmaGenesis and Shire, employees of which reviewed and approved the draft text.