Data Sharing Statement Reporting Across Medical Specialties
Abstract
Eli Paul,1 Griffin Hughes,1 Alex Hagood,1 Matt Vassar1,2
Objective
Data sharing promotes secondary use, transparency, and reproducibility, yet prior studies have revealed gaps in policy standardization and implementation.1 Although some evaluations have explored data sharing statement (DSS) practices within specific fields or policy contexts, no broad, comparative assessment across medical specialties exists.2,3 This meta-analysis assesses DSS prevalence across 19 medical specialties and examines variability in adoption rates.
Design
We conducted a meta-analysis using harmonized article-level data from 19 separate studies, each prospectively designed and executed by our research team using the same methodology, with each study focused on a distinct medical specialty. Between December 2023 and July 2024, all studies systematically searched PubMed for original research articles published between 2018 and 2023. Study designs included in our study were clinical trials, cohort studies, cross-sectional studies, case series, case-control studies, cost-effectiveness analyses, qualitative research, survey-based studies, and other designs that involved human participants. Reviews, editorials, and commentaries were excluded. For each article, we assessed the presence of a DSS and whether data were publicly accessible. Two independent reviewers performed screening and data extraction per specialty, with discrepancies resolved by consensus. We conducted a random-effects meta-analysis using inverse-variance weights based on standard errors of DSS proportions. Influential study diagnostics were applied to identify and mitigate the impact of extreme specialty effects. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline was followed for the design and execution of our study.
Results
Among 20,095 eligible articles, 6092 (30.3%) reported a DSS (Figure 25-1134). Prevalence varied substantially by specialty, from 63.9% in Rheumatology to 1.5% in Plastic Surgery. The pooled DSS reporting rate was 29.8% (prediction interval, 0-65.9%), with high heterogeneity (I² = 99.60%). After removing influential specialties (rheumatology, neurology, and plastic surgery), the adjusted pooled rate was 27.5% (prediction interval, 0-54.9%), with high heterogeneity persisting (I² = 99.14%).
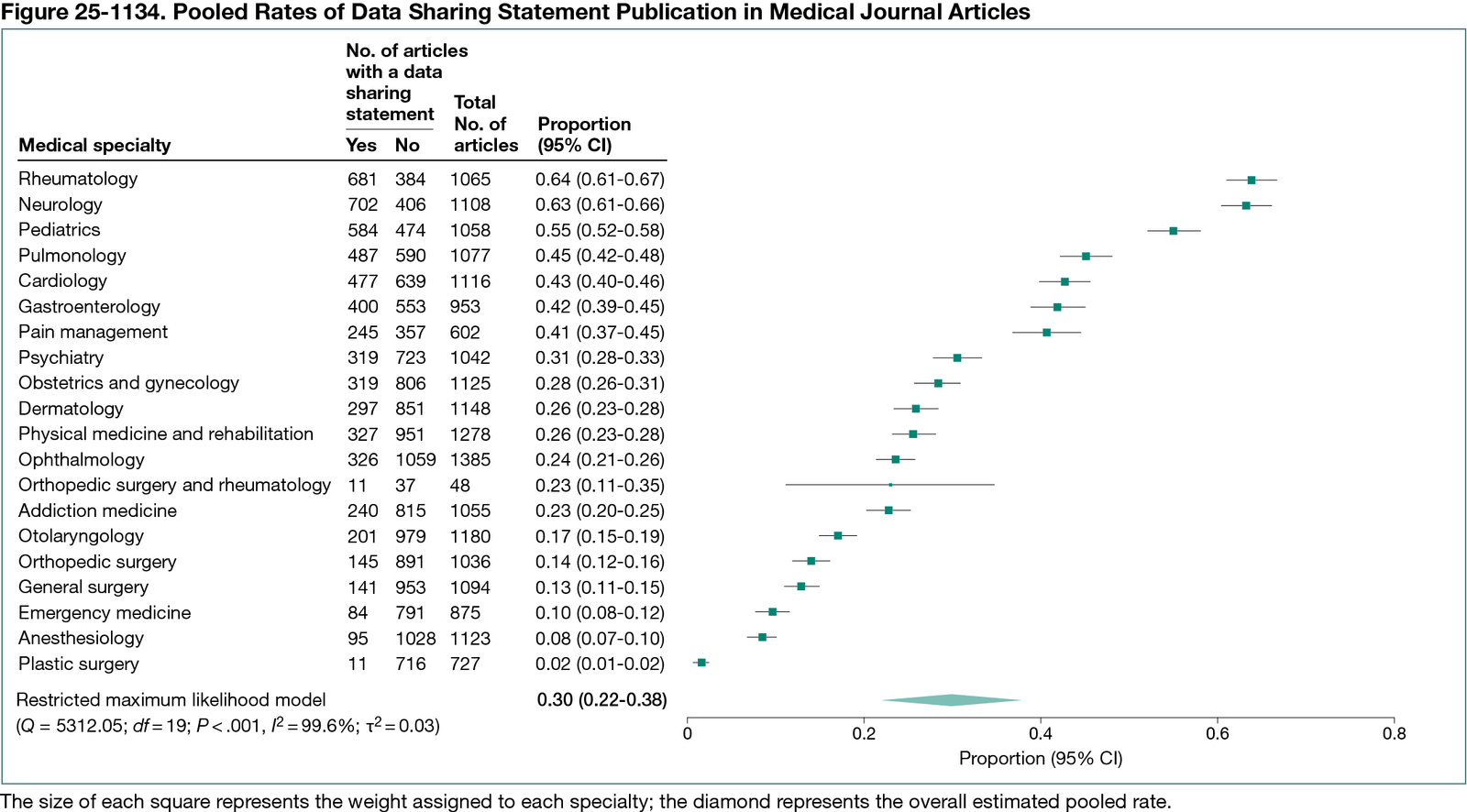
Conclusions
Despite increasing emphasis on open science, DSS reporting remains low and inconsistent across medical specialties. The high heterogeneity suggests that DSS reporting is shaped more by specialty-specific norms and editorial practices than by standardized policy. By conducting 19 internally coordinated studies using identical methodology, our team generated a uniquely standardized dataset that enabled robust, cross-specialty comparisons. Stronger policy enforcement and cross-disciplinary coordination among journals, funders, and regulatory bodies are needed to improve transparency and foster responsible data sharing in medical research.
References
1. Johnson AL, Anderson JM, Bouvette M, et al. Clinical trial data-sharing policies among journals, funding agencies, foundations, and other professional organizations: a scoping review. J Clin Epidemiol. 2023;154:42-55. doi:10.1016/j.jclinepi.2022.11.009
2. Tedersoo L, Küngas R, Oras E, et al. Data sharing practices and data availability upon request differ across scientific disciplines. Sci Data. 2021;8:192. doi:10.1038/s41597-021-00981-0
3. Vassar M, Jellison S, Wendelbo H, Wayant C. Data sharing practices in randomized trials of addiction interventions. Addict Behav. 2020;102:106193. doi:10.1016/j.addbeh.2019.106193
1Office of Medical Student Research, Oklahoma State University Center for Health Sciences, Tulsa, OK, US, eli.paul@okstate.edu; 2Department of Psychiatry and Behavioral Sciences, Oklahoma State University Center for Health Sciences, Tulsa, OK, US.
Conflict of Interest Disclosures
Matt Vassar reported receiving funding from the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, the US Office of Research Integrity, and the Oklahoma Center for Advancement of Science and Technology and internal grants from the Oklahoma State University Center for Health Sciences outside the submitted work. No other disclosures were reported.
