Conflicts of Interest in Research Across Scholarly Disciplines
Abstract
Helena Van Beersel Krejcikova,1,2 Christoffer Bruun Korfitsen,1,2 Lisa Bero,3 Jason Dana,4 David C. Dorman,5 Quinn Grundy,6 Ibo van de Poel,7 Morten Rosenmeier,8 Asbjørn Hróbjartsson,1,2 Andreas Lundh1,2,9
Objective
Conflicts of interest can undermine the trustworthiness of research if they are not adequately prevented or managed. Our objective was to (1) map and analyze conflict of interest policies of important international and national scholarly organizations within 5 selected scholarly disciplines and (2) describe cross-disciplinary differences and commonalities.
Design
This was a cross-sectional study and content analysis of conflict of interest policies for research in economics, engineering, environmental toxicology and chemistry, law, and medicine. We used purposive sampling to include international and national policies in English from: (1) 100 top scholarly journals (20 per discipline) and the 20 largest publishers (both based on rankings in Clarivate’s Journal Citation Reports 2022); (2) major journal and publisher associations and international multidisciplinary scholarly organizations (identified via targeted web search); (3) top universities (2 per continent based on ranking in Times Higher Education World University Rankings 2023); and (4) public funding agencies with the largest amount of annual funding (up to 2 per continent and discipline, identified via targeted web search). One researcher identified potentially eligible policies (April 2025), and 2 researchers independently included policies and extracted data. In this preliminary analysis, we report descriptive statistics stratified by type of scholarly discipline and organization. The protocol and study registration can be found online.1
Results
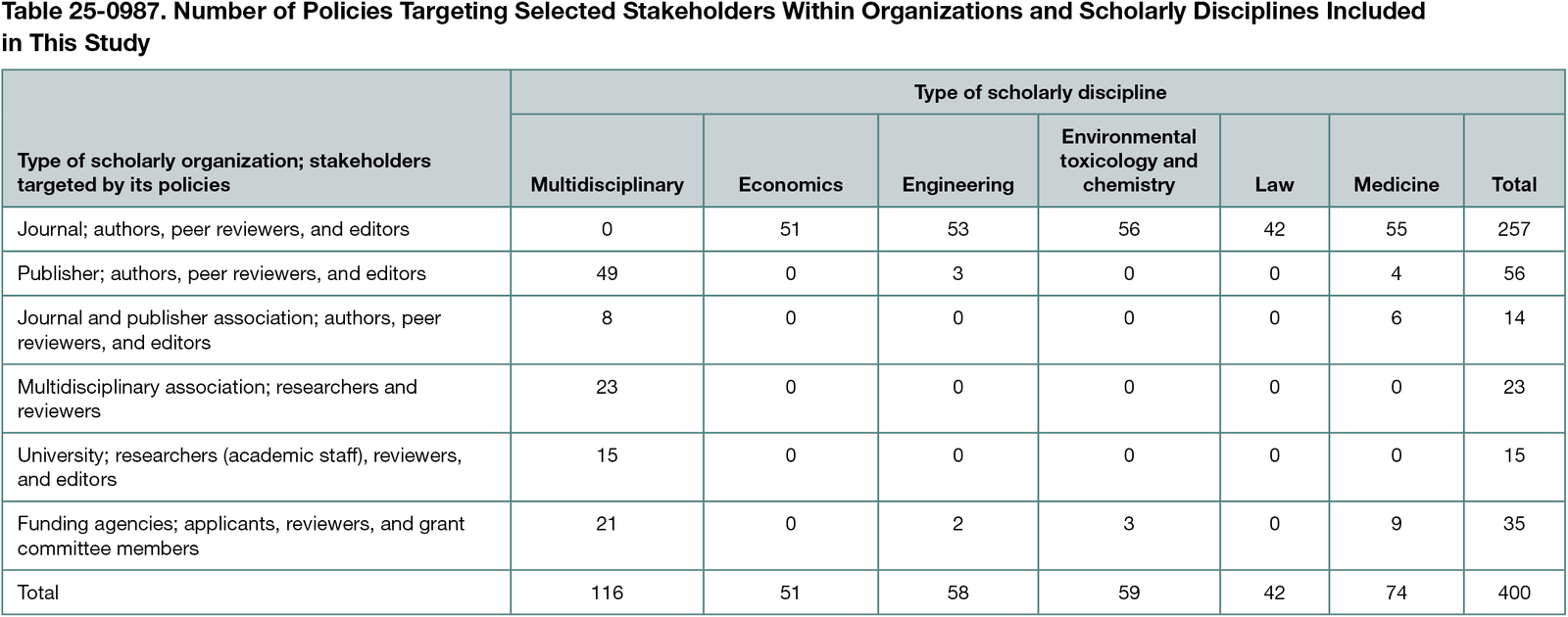
We screened 1008 organizations and included policies from 170 scholarly organizations: 100 journals, 20 publishers, 6 journal and publisher associations, 14 international multidisciplinary scholarly associations, 12 universities, and 18 funding agencies. From these organizations, we included 400 policies targeting different stakeholders (Table 25-0987): 159 policies (40%) relevant for researchers (eg, manuscript authors or grant applicants), 126 (32%) for peer reviewers, and 115 (29%) for editors or grant committee members. A total of 369 policies (92%) addressed both financial and other interests, 13 (3%) addressed exclusively financial interests, and 18 (5%) addressed exclusively other interests. A total of 350 policies (88%) addressed disclosure of interests and 287 (72%) addressed the management of conflicts of interest. Content describing both disclosure requirements and management of disclosed conflicts of interest could be found in 71 of 159 policies (45%) for researchers, 87 of 126 (69%) for peer reviewers, and 79 of 115 (69%) for editors or grant committee members. Enforcement strategies (eg, consequences of violating policies) to ensure the compliance with both disclosure and management requirements were addressed in 16 of 159 policies (10%) for researchers, 19 of 126 (15%) for peer reviewers, and 7 of 115 (6%) for editors or grant committee members.
Conclusions
Most of the top scholarly organizations have conflict of interest policies addressing both financial and other types of interests. While almost all policies addressed disclosure of conflicts of interest, only slightly more than one-half addressed both disclosure of conflicts of interest and how disclosed conflicts of interest should be managed. Few policies described how they should be enforced.
Reference
1. Krejcikova HVB, Korfitsen CB, Bero L, et al. Conflicts of interest in research across scholarly disciplines: cross-sectional study and qualitative content analysis of policies. October 16, 2023. Accessed July 15, 2023. https://osf.io/vwn6c
1Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark, hkrejcikova@health.sdu.dk; 2Open Patient Data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark; 3Center for Bioethics and Humanities, University of Colorado Anschutz Medical Campus, Aurora, CO, US; 4Yale School of Management, New Haven, CT, US; 5North Carolina State University College of Veterinary Medicine, Raleigh, NC, US; 6Lawrence Bloomberg Faculty of Nursing, University of Toronto, Toronto, Ontario, Canada; 7School of Technology, Policy and Management, Delft University of Technology, Delft, the Netherlands; 8Centre for Information and Innovation Law (CIIR), Faculty of Law, University of Copenhagen, Copenhagen, Denmark; 9Department of Respiratory Medicine and Infectious Diseases, Copenhagen University Hospital–Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Conflict of Interest Disclosures
Lisa Bero is a meta-research section editor for PLoS Biology, a Conflict of Interest Advisor for Health Canada, and a Senior Research Integrity Editor at Cochrane, for which the University of Colorado receives remuneration. David C. Dorman is an associate editor for Animals and Critical Reviews in Toxicology. Ibo van de Poel is the integrity officer of the Delft University of Technology; sits on the editorial boards of Science and Engineering Ethics, AI and Ethics, and International Journal of Technoethics; and contributed to the book series SpringerBriefs on Ethical and Legal Issues in Biomedicine and Technology. Asbjørn Hróbjartsson is an associate editor of the Journal of Evidence-Based Medicine and the Cochrane Methodology Review Group and sits on the editorial board of the Journal of Clinical Epidemiology. Andreas Lundh sits on the editorial board of BMC Medical Ethics. Quinn Grundy is a member of the Peer Review Congress Advisory Board but was not involved in the review or decision for this abstract.
