Abstract
Presence of a Unique Trial Identifier in the Abstracts of Industry-Sponsored Manuscripts
LaVerne A. Mooney,1 Joseph F. Michalski,2 Lorna Fay1
Objective
Since 2008, the International Committee of Medical Journal Editors (ICMJE) has recommended that journals include a unique trial identifier (eg, the Clinical.Trials.gov ID number [NCT]) in the abstract of clinical trial manuscripts. Adherence to this recommendation should result in automatic linkage between the PubMed abstract and the trial record on ClinicalTrials.gov, and inclusion of the manuscript citation in the trial record. A 2013 study reported that unique trial identifiers are frequently missing from published manuscript abstracts. Our objective was to assess the implementation of the ICMJE’s recommendation in manuscripts reporting results of Pfizer-sponsored trials and determine whether the relevant citation was present on ClinicalTrials.gov.
Design
Using a Pfizer publication database, we identified manuscripts reporting primary outcomes for Pfizer clinical trials published from 2013 to 2015, and we obtained corresponding NCT numbers from ClinicalTrials.gov. We excluded manuscripts of trials that were not indexed on PubMed, that were not registered on ClinicalTrials.gov, or that reported non-interventional studies. For each clinical trial, we recorded the presence or absence of the NCT number in the manuscript PDF and its location within the manuscript, the presence or absence of the NCT number in the PubMed abstract, and the presence or absence of a manuscript citation on the trial record and if it had been automatically indexed or added by the sponsor. We report percentages overall and by year.
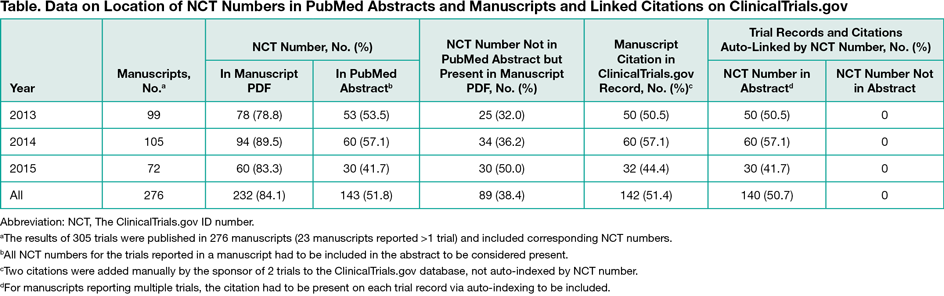
Results
A total of 276 manuscripts (305 studies) were published in 140 unique journals. Half of the PubMed abstracts (143 of 276 [51.8%]) included the NCT number, and the citation was present in 142 of 276 (51.4%) of the ClinicalTrial.gov records (Table). Auto-indexing accounted for 140 of 142 (99.0%) of the citations on trial records; only 2 resulted from manual addition by the sponsor to the study records when the NCT number was not present in the abstracts. Errors or failures of the auto-indexing process appeared to occur for 3 abstracts published in 2013.
Conclusions
The NCT number was included in most Pfizer clinical trial manuscripts (232 of 276 [84.1%]), but auto-indexing and bidirectional linkage (from PubMed and from ClinicalTrials.gov) only occurred when the NCT number was located in the abstract per ICMJE guidelines. An additional 89 manuscript citations could have been visible to the public on ClinicalTrials.gov if the NCT number was correctly positioned in the abstract. This study demonstrates the importance of the location of the trial identifier within the manuscript.
1Pfizer Medical, New York, NY, USA, laverne.mooney@pfizer.com; 2Mailman School of Public Health, Health Policy and Management, Columbia University, New York, NY, USA
Conflict of Interest Disclosures:
L. Mooney and L. Fay are employees of Pfizer Inc and hold Pfizer stock. J. Michalski is a graduate student who assisted with the research, was paid by Pfizer, and holds Pfizer stock. No other disclosures were reported.
Funding/Support:
Pfizer is the employer of two of the authors and the analysis was carried out by them at Pfizer.