Abstract
Adverse Event Reporting in Registered and Published Clinical Trials Focusing on Drug-Drug Interactions
Diana Jurić,1 Shelly Pranić,2 Ivančica Pavličević,3 Ana Marušić4
Objective
Drug-drug interactions (DDIs) are a growing concern because of rising numbers of chemical entities and more prevalent polypharmacy. Several market withdrawals due to interaction-related adverse events (AEs) suggest the importance of reporting AEs in clinical studies focusing on DDIs. The aim of this study was to compare the characteristics of AEs in clinical trials focusing on DDIs registered in ClinicalTrials.gov and subsequently published in the medical literature.
Design
As part of a larger study, we retrieved clinical trials focusing on DDIs from ClinicalTrials.gov using the search term drug-drug interaction(s) on October 16, 2015, and collected data on AEs from corresponding publications. Trials were included if they (1) primarily investigated DDIs; (2) had a ClinicalTrials.gov NCT number; (3) were registered between June 23, 2005, and October 16, 2015; and (4) were closed and completed by October 16, 2015. Published articles and their online supplementary material were identified from ClinicalTrials.gov and PubMed using the [si] tag along with the NCT number, and from SCOPUS/EMBASE using the first author’s name and the study title. Data were abstracted by one author and verified by a second.
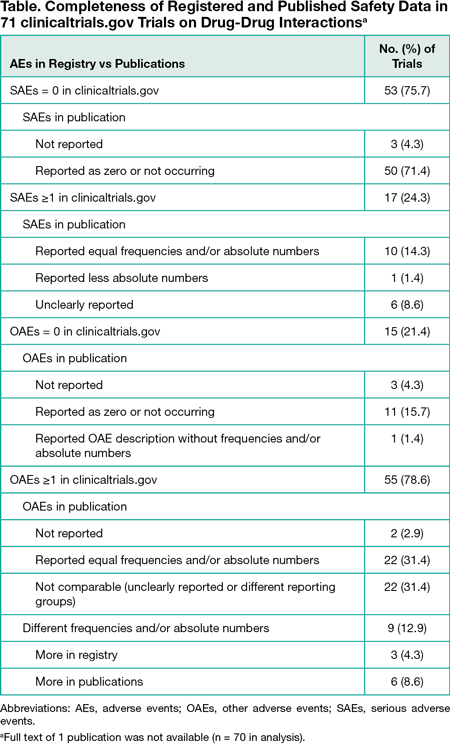
Results
Among 2059 retrieved trials, 762 (37.0%) were excluded because they were not related to DDIs or had a change in the trial status. Of the remaining 1297 trials, most were interventional (1244 [95.9%]), industry funded (845 [65.2%]), and started before registration (745 [57.4%]). Trials were commonly phase 1 (895 [69.0%]), included healthy volunteers (873 [67.3%]), and had pharmacokinetic measures as the most common primary end point (633 [48.8%]). Results were registered for 164 trials (12.6%), among which 71 (43.3%) were published. However, the full text of 1 published trial was not available, leaving 70 trials in the analysis (Table). Published data about both serious and other AEs were identical to registered data for 17 trials (24.3%). Three trials (4.3%) with registered safety data did not describe them in publications. In 55 published trials with 1 or more other AEs recorded, equal absolute numbers and/or frequencies as in the registry were clearly reported for only 22 (31.4%). Different numbers of participants who discontinued treatment due to an AE were registered and reported for 2 trials.
Conclusions
There are discrepancies between registered and published AE data for trials focusing on DDIs that emerge from incomplete or changed reporting of AEs in publications. There is a need to enforce regulatory requirements for timely and complete registration of results, and a need for clearer AE reporting for trials focusing on DDIs, including phase 1 trials, and for assessment of the congruence of registered and submitted AE data during the publication process.
1Department of Pharmacology, University of Split School of Medicine, Split, Croatia, diana.juric26@gmail.com; 2Departments of Research in Biomedicine and Health and Public Health, University of Split School of Medicine, Split, Croatia; 3Department of Family Medicine, University of Split School of Medicine, Split, Croatia, 4Department of Research in Biomedicine and Health, University of Split School of Medicine, Split, Croatia
Conflict of Interest Disclosures:
Dr Marušić is a Peer Review Congress Advisory Board Member but was not involved in the review or decision for this abstract. No other disclosures were reported.
Funding/Support:
This study was funded by grant IP-2014-09-7672 (“Professionalism in Health Care”) from the Croatian Science Foundation. The funder had no role in the design of this study or during its execution and data interpretation.